A new resistance gene, linB, conferring resistance to lincosamides by nucleotidylation in Enterococcus faecium HM1025
- PMID: 10103201
- PMCID: PMC89227
- DOI: 10.1128/AAC.43.4.925
A new resistance gene, linB, conferring resistance to lincosamides by nucleotidylation in Enterococcus faecium HM1025
Abstract
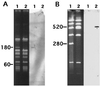
Resistance to lincomycin and clindamycin in the clinical isolate Enterococcus faecium HM1025 is due to a ribosomal methylase encoded by an ermAM-like gene and the plasmid-mediated inactivation of these antibiotics. We have cloned and determined the nucleotide sequence of the gene responsible for the inactivation of lincosamides, linB. This gene encodes a 267-amino-acid lincosamide nucleotidyltransferase. The enzyme catalyzes 3(5'-adenylation) (the adenylation of the hydroxyl group in position 3 of the molecules) of lincomycin and clindamycin. Expression of linB was observed in both Escherichia coli and Staphylococcus aureus. The deduced amino acid sequence of the enzyme did not display any significant homology with staphylococcal nucleotidyltransferases encoded by linA and linA' genes. Sequences homologous to linB were found in 14 other clinical isolates of E. faecium, indicating the spread of the resistance trait in this species.
Figures

References
-
- Argoudelis A D, Coats J H. Microbial transformation of antibiotics. II. Phosphorylation of lincomycin by streptomyces species. J Antibiot. 1969;22:341–343. - PubMed
-
- Argoudelis A D, Coats J H, Mizsak S A. Microbial transformation of antibiotics. Clindamycin ribonucleotides. J Antibiot. 1977;30:474–487. - PubMed
-
- Bouanchaud H C, Scavizzi M R, Chabbert Y A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1969;54:417–425. - PubMed
-
- Brisson-Noël A, Courvalin P. Nucleotide sequence of gene linA encoding resistance to lincosamides in Staphylococcus haemolyticus. Gene. 1986;43:247–253. - PubMed
-
- Brisson-Noël A, Delrieu P, Samain D, Courvalin P. Inactivation of lincosaminide antibiotics in Staphylococcus. Identification of lincosaminide O-nucleotidyltransferases and comparison of the corresponding resistance genes. J Biol Chem. 1988;263:15880–15887. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

