Initial reactions in the biodegradation of 1-chloro-4-nitrobenzene by a newly isolated bacterium, strain LW1
- PMID: 10103229
- PMCID: PMC91199
- DOI: 10.1128/AEM.65.4.1405-1412.1999
Initial reactions in the biodegradation of 1-chloro-4-nitrobenzene by a newly isolated bacterium, strain LW1
Abstract
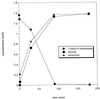
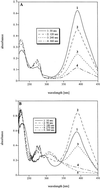
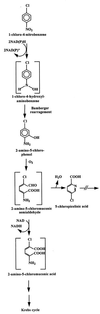
Bacterial strain LW1, which belongs to the family Comamonadaceae, utilizes 1-chloro-4-nitrobenzene (1C4NB) as a sole source of carbon, nitrogen, and energy. Suspensions of 1C4NB-grown cells removed 1C4NB from culture fluids, and there was a concomitant release of ammonia and chloride. Under anaerobic conditions LW1 transformed 1C4NB into a product which was identified as 2-amino-5-chlorophenol by 1H and 13C nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry. This transformation indicated that there was partial reduction of the nitro group to the hydroxylamino substituent, followed by Bamberger rearrangement. In the presence of oxygen but in the absence of NAD, fast transformation of 2-amino-5-chlorophenol into a transiently stable yellow product was observed with resting cells and cell extracts. This compound exhibited an absorption maximum at 395 nm and was further converted to a dead-end product with maxima at 226 and 272 nm. The compound formed was subsequently identified by 1H and 13C NMR spectroscopy and mass spectrometry as 5-chloropicolinic acid. In contrast, when NAD was added in the presence of oxygen, only minor amounts of 5-chloropicolinic acid were formed, and a new product, which exhibited an absorption maximum at 306 nm, accumulated.
Figures



Similar articles
-
Degradation of nitrobenzene by a Pseudomonas pseudoalcaligenes.Appl Environ Microbiol. 1993 Aug;59(8):2520-5. doi: 10.1128/aem.59.8.2520-2525.1993. Appl Environ Microbiol. 1993. PMID: 8368838 Free PMC article.
-
Aerobic degradation of 2-picolinic acid by a nitrobenzene-assimilating strain: Streptomyces sp. Z2.Bioresour Technol. 2009 Mar;100(6):2082-4. doi: 10.1016/j.biortech.2008.10.017. Epub 2008 Nov 29. Bioresour Technol. 2009. PMID: 19042125
-
Bacterial dehalogenation of chlorobenzoates and coculture biodegradation of 4,4'-dichlorobiphenyl.Appl Environ Microbiol. 1989 Apr;55(4):887-92. doi: 10.1128/aem.55.4.887-892.1989. Appl Environ Microbiol. 1989. PMID: 2499257 Free PMC article.
-
Biodegradation of nitroaromatic compounds.Annu Rev Microbiol. 1995;49:523-55. doi: 10.1146/annurev.mi.49.100195.002515. Annu Rev Microbiol. 1995. PMID: 8561470 Review.
-
Evolution of catabolic pathways for synthetic compounds: bacterial pathways for degradation of 2,4-dinitrotoluene and nitrobenzene.Appl Microbiol Biotechnol. 2003 Aug;62(2-3):110-23. doi: 10.1007/s00253-003-1341-4. Epub 2003 May 15. Appl Microbiol Biotechnol. 2003. PMID: 12750857 Review.
Cited by
-
Novel partial reductive pathway for 4-chloronitrobenzene and nitrobenzene degradation in Comamonas sp. strain CNB-1.Appl Environ Microbiol. 2006 Mar;72(3):1759-65. doi: 10.1128/AEM.72.3.1759-1765.2006. Appl Environ Microbiol. 2006. PMID: 16517619 Free PMC article.
-
The Enzymology of Organic Transformations: A Survey of Name Reactions in Biological Systems.Angew Chem Int Ed Engl. 2017 Mar 20;56(13):3446-3489. doi: 10.1002/anie.201603291. Epub 2017 Feb 14. Angew Chem Int Ed Engl. 2017. PMID: 27505692 Free PMC article. Review.
-
Members of the family Comamonadaceae as primary poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-degrading denitrifiers in activated sludge as revealed by a polyphasic approach.Appl Environ Microbiol. 2002 Jul;68(7):3206-14. doi: 10.1128/AEM.68.7.3206-3214.2002. Appl Environ Microbiol. 2002. PMID: 12088996 Free PMC article.
-
Reactions involved in the lower pathway for degradation of 4-nitrotoluene by Mycobacterium strain HL 4-NT-1.Appl Environ Microbiol. 2000 Jul;66(7):3010-5. doi: 10.1128/AEM.66.7.3010-3015.2000. Appl Environ Microbiol. 2000. PMID: 10877799 Free PMC article.
-
Nitroaromatic compounds, from synthesis to biodegradation.Microbiol Mol Biol Rev. 2010 Jun;74(2):250-72. doi: 10.1128/MMBR.00006-10. Microbiol Mol Biol Rev. 2010. PMID: 20508249 Free PMC article. Review.
References
-
- Aoki K, Takenaka S, Murakami S, Shinke R. Partial purification and characterization of a bacterial dioxygenase that catalyzes the ring fission of 2-aminophenol. Microbiol Rev. 1997;152:33–38.
-
- Asano Y, Yamamoto Y, Yamada H. Catechol 2,3-dioxygenase-catalyzed synthesis of picolinic acids from catechols. Biosci Biotechnol Biochem. 1994;58:2054–2056.
-
- Beil S, Happe B, Timmis K N, Pieper D H. Genetic and biochemical characterization of the broad-spectrum chlorobenzene dioxygenase from Burkholderia sp. strain PS12: dechlorination of 1,2,4,5-tetrachlorobenzene. Eur J Biochem. 1997;247:190–199. - PubMed
-
- Beratergremium für umweltrelevante Altstoffe der Gesellschaft Deutscher Chemiker. m-Chlornitrobenzol, p-Chlornitrobenzol. BUA-Stoffbericht 11 (Februar 1988). Weinheim, Germany: VCH Verlagsgesellschaft; 1988.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

