Integrative model for binding of Bacillus thuringiensis toxins in susceptible and resistant larvae of the diamondback moth (Plutella xylostella)
- PMID: 10103230
- PMCID: PMC91200
- DOI: 10.1128/AEM.65.4.1413-1419.1999
Integrative model for binding of Bacillus thuringiensis toxins in susceptible and resistant larvae of the diamondback moth (Plutella xylostella)
Abstract
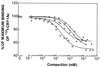
Insecticidal crystal proteins from Bacillus thuringiensis in sprays and transgenic crops are extremely useful for environmentally sound pest management, but their long-term efficacy is threatened by evolution of resistance by target pests. The diamondback moth (Plutella xylostella) is the first insect to evolve resistance to B. thuringiensis in open-field populations. The only known mechanism of resistance to B. thuringiensis in the diamondback moth is reduced binding of toxin to midgut binding sites. In the present work we analyzed competitive binding of B. thuringiensis toxins Cry1Aa, Cry1Ab, Cry1Ac, and Cry1F to brush border membrane vesicles from larval midguts in a susceptible strain and in resistant strains from the Philippines, Hawaii, and Pennsylvania. Based on the results, we propose a model for binding of B. thuringiensis crystal proteins in susceptible larvae with two binding sites for Cry1Aa, one of which is shared with Cry1Ab, Cry1Ac, and Cry1F. Our results show that the common binding site is altered in each of the three resistant strains. In the strain from the Philippines, the alteration reduced binding of Cry1Ab but did not affect binding of the other crystal proteins. In the resistant strains from Hawaii and Pennsylvania, the alteration affected binding of Cry1Aa, Cry1Ab, Cry1Ac, and Cry1F. Previously reported evidence that a single mutation can confer resistance to Cry1Ab, Cry1Ac, and Cry1F corresponds to expectations based on the binding model. However, the following two other observations do not: the mutation in the Philippines strain affected binding of only Cry1Ab, and one mutation was sufficient for resistance to Cry1Aa. The imperfect correspondence between the model and observations suggests that reduced binding is not the only mechanism of resistance in the diamondback moth and that some, but not all, patterns of resistance and cross-resistance can be predicted correctly from the results of competitive binding analyses of susceptible strains.
Figures



References
-
- Ballester V, Escriche B, Ménsua J L, Riethmacher G W, Ferré J. Lack of cross-resistance to other Bacillus thuringiensis crystal proteins in a population of Plutella xylostella highly resistant to CryIA(b) Biocontrol Sci Technol. 1994;4:437–443.
-
- Bauer L S. Resistance: a threat to the insecticidal crystal proteins of Bacillus thuringiensis. Fla Entomol. 1995;78:414–443.
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Chen X J, Curtiss A, Alcantara E, Dean D H. Mutations in domain I of Bacillus thuringiensis δ-endotoxin CryIAb reduce the irreversible binding of toxin to Manduca sexta brush border membrane vesicles. J Biol Chem. 1995;270:6412–6419. - PubMed
-
- Escriche B, Silva F J, Ferré J. Testing suitability of brush border membrane vesicles prepared from whole larvae from small insects for binding studies with Bacillus thuringiensis CryIA(b) crystal protein. J Invert Pathol. 1995;65:318–320.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

