Effects of vasopressin on the sympathetic contraction of rabbit ear artery during cooling
- PMID: 10188992
- PMCID: PMC1565852
- DOI: 10.1038/sj.bjp.0702345
Effects of vasopressin on the sympathetic contraction of rabbit ear artery during cooling
Abstract
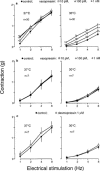
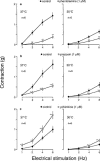
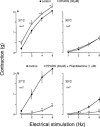
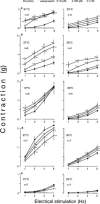
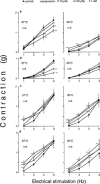
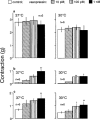
In order to analyse the effects of arginine-vasopressin on the vascular contraction to sympathetic nerve stimulation during cooling, the isometric response of isolated, 2-mm segments of the rabbit central ear (cutaneous) artery to electrical field stimulation (1-8 Hz) was recorded at 37 and 30 degrees C. Electrical stimulation (37 degrees C) produced frequency-dependent arterial contraction, which was reduced at 30 degrees C and potentiated by vasopressin (10 pM, 100 pM and 1 nM). This potentiation was greater at 30 than at 37 degrees C and was abolished at both temperatures by the antagonist of vasopressin V1 receptors d(CH2)5 Tyr(Me)AVP (100 nM). Desmopressin (1 microM) did not affect the response to electrical stimulation. At 37 degrees C, the vasopressin-induced potentiation was abolished by the purinoceptor antagonist PPADS (30 microM), increased by phentolamine (1 microM) or prazosin (1 microM) and not modified by yohimbine (1 microM), whilst at 30 degrees C, the potentiation was reduced by phentolamine, yohimbine or PPADS, and was not modified by prazosin. The Ca2+-channel blockers, verapamil (10 microM) and NiCl2 (1 mM), abolished the potentiating effects of vasopressin at 37 degrees C whilst verapamil reduced and NiCl2 abolished this potentiation at 30 degrees C. The inhibitor of nitric oxide synthesis, L-NOARG (100 microM), or endothelium removal did not modify the potentiation by vasopressin at 37 and 30 degrees C. Vasopressin also increased the arterial contraction to the alpha2-adrenoceptor agonist BHT-920 (10 microM) and to ATP (2 mM) at 30 and 37 degrees C, but it did not modify the contraction to noradrenaline (1 microM) at either temperature. These results suggest that in cutaneous (ear) arteries, vasopressin potentiaties sympathetic vasoconstriction to a greater extent at 30 than at 37 degrees C by activating vasopressin V1 receptors and Ca2+ channels at both temperatures. At 37 degrees C, the potentiation appears related to activation of the purinoceptor component and, at 30 degrees C, to activation of both purinoceptor and alpha2-adrenoceptor components of the sympathetic response.
Figures

Similar articles
-
Role of the purinergic and noradrenergic components in the potentiation by endothelin-1 of the sympathetic contraction of the rabbit central ear artery during cooling.Br J Pharmacol. 1997 Sep;122(1):172-8. doi: 10.1038/sj.bjp.0701359. Br J Pharmacol. 1997. PMID: 9298544 Free PMC article.
-
Effect of neuropeptide Y on the sympathetic contraction of the rabbit central ear artery during cooling.Pflugers Arch. 2000 Aug;440(4):548-55. doi: 10.1007/s004240000323. Pflugers Arch. 2000. PMID: 10958338
-
Role of endothelin receptors, calcium and nitric oxide in the potentiation by endothelin-1 of the sympathetic contraction of rabbit ear artery during cooling.Br J Pharmacol. 1997 Aug;121(8):1659-64. doi: 10.1038/sj.bjp.0701324. Br J Pharmacol. 1997. PMID: 9283700 Free PMC article.
-
[Characterization of the facilitatory modulation of adrenergic neurotransmission via prejunctional purinoceptors in rabbit ear artery].Yakugaku Zasshi. 1999 Jun;119(6):417-28. doi: 10.1248/yakushi1947.119.6_417. Yakugaku Zasshi. 1999. PMID: 10376002 Review. Japanese.
-
Adrenergic mechanisms in the rabbit ear artery. A review.Blood Vessels. 1975;12(3):137-60. doi: 10.1159/000158046. Blood Vessels. 1975. PMID: 1100143 Review.
Cited by
-
Role of alpha2C-adrenoceptors in the reduction of skin blood flow induced by local cooling in mice.Br J Pharmacol. 2007 Sep;152(1):91-100. doi: 10.1038/sj.bjp.0707380. Epub 2007 Jul 9. Br J Pharmacol. 2007. PMID: 17618305 Free PMC article.
-
Efficacy of cooling therapy and α-lipoic acid derivative against chemotherapy-induced alopecia in an animal model.Cancer Sci. 2023 Mar;114(3):1007-1014. doi: 10.1111/cas.15639. Epub 2022 Nov 15. Cancer Sci. 2023. PMID: 36337052 Free PMC article.
References
-
- ALTURA B.M., ALTURA B.T. Vascular smooth muscle and neurohypophyseal hormones. Fed. Proc. 1977;36:1853–1860. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

