Axons from anteroventral cochlear nucleus that terminate in medial superior olive of cat: observations related to delay lines
- PMID: 10191328
- PMCID: PMC6782263
- DOI: 10.1523/JNEUROSCI.19-08-03146.1999
Axons from anteroventral cochlear nucleus that terminate in medial superior olive of cat: observations related to delay lines
Abstract
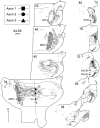
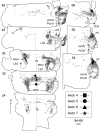
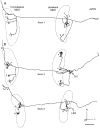
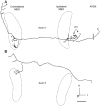
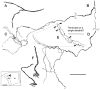
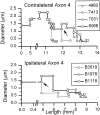
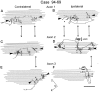
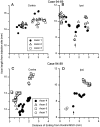
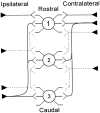
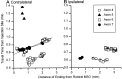
The differences in path length of axons from the anteroventral cochlear nuclei (AVCN) to the medial superior olive (MSO) are thought to provide the anatomical substrate for the computation of interaural time differences (ITD). We made small injections of biotinylated dextran into the AVCN that produced intracellular-like filling of axons. This permitted three-dimensional reconstructions of individual axons and measurements of axonal length to individual terminals in MSO. Some axons that innervated the contralateral MSO had collaterals with lengths that were graded in the rostrocaudal direction with shorter collaterals innervating more rostral parts of MSO and longer collaterals innervating more caudal parts of MSO. These could innervate all or part of the length of the MSO. Other axons had restricted terminal fields comparable to the size of a single dendritic tree in the MSO. In the ipsilateral MSO, some axons had a reverse, but less steep, gradient in axonal length with greater axonal length associated with more rostral locations; others had restricted terminal fields. Thus, the computation of ITDs is based on gradients of axonal length in both the contralateral and ipsilateral MSO, and these gradients may account for a large part of the range of ITDs encoded by the MSO. Other factors may be involved in the computation of ITDs to compensate for differences between axons.
Figures



References
-
- Batra R, Kuwada S, Fitzpatrick DC. Sensitivity to interaural temporal disparities of low- and high-frequency neurons in the superior olivary complex: I. Heterogeneity of responses. J Neurophysiol. 1997a;78:1222–1236. - PubMed
-
- Batra R, Kuwada S, Fitzpatrick DC. Sensitivity to interaural temporal disparities of low- and high-frequency neurons in the superior olivary complex: II. Coincidence detection. J Neurophysiol. 1997b;78:1237–1247. - PubMed
-
- Bernstein LR, Trahiotis C. The normalized correlation: Accounting for binaural detection across center frequency. J Acoust Soc Am. 1996;100:3774–3784. - PubMed
-
- Cant NB. The cochlear nucleus: neuronal types and their synaptic organization. In: Webster DB, Popper AN, Ray RR, editors. The Mammalian auditory pathway: neuroanatomy, Vol 1. Springer; New York: 1992. pp. 66–116.
-
- Cant NB, Hyson RL. Projections from the lateral nucleus of the trapezoid body to the medial superior olivary nucleus in the gerbil. Hear Res. 1992;58:26–34. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
