Hypothalamic hypocretin (orexin): robust innervation of the spinal cord
- PMID: 10191330
- PMCID: PMC6782271
- DOI: 10.1523/JNEUROSCI.19-08-03171.1999
Hypothalamic hypocretin (orexin): robust innervation of the spinal cord
Abstract
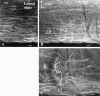
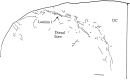
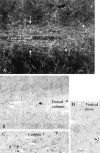
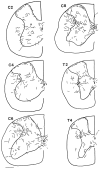
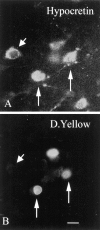
Hypocretin (orexin) is synthesized by neurons in the lateral hypothalamus and has been reported to increase food intake and regulate the neuroendocrine system. In the present paper, long descending axonal projections that contain hypocretin were found that innervate all levels of the spinal cord from cervical to sacral segments, as studied in mouse, rat, and human spinal cord and not previously described. High densities of axonal innervation are found in regions of the spinal cord related to modulation of sensation and pain, notably in the marginal zone (lamina 1). Innervation of the intermediolateral column and lamina 10 as well as strong innervation of the caudal region of the sacral cord suggest that hypocretin may participate in the regulation of both the sympathetic and parasympathetic parts of the autonomic nervous system. Double-labeling experiments in mice combining retrograde transport of diamidino yellow after spinal cord injections and immunocytochemistry support the concept that hypocretin-immunoreactive fibers in the cord originate from the neurons in the lateral hypothalamus. Digital-imaging physiological studies with fura-2 detected a rise in intracellular calcium in response to hypocretin in cultured rat spinal cord neurons, indicating that spinal cord neurons express hypocretin-responsive receptors. A greater number of cervical cord neurons responded to hypocretin than another hypothalamo-spinal neuropeptide, oxytocin. These data suggest that in addition to possible roles in feeding and endocrine regulation, the descending hypocretin fiber system may play a role in modulation of sensory input, particularly in regions of the cord related to pain perception and autonomic tone.
Figures






Similar articles
-
Lateral hypothalamus: early developmental expression and response to hypocretin (orexin).J Comp Neurol. 2001 May 7;433(3):349-63. doi: 10.1002/cne.1144. J Comp Neurol. 2001. PMID: 11298360
-
Distribution of orexin-A and orexin-B (hypocretins) in the rat spinal cord.Neurosci Lett. 2000 Jul 14;288(2):87-90. doi: 10.1016/s0304-3940(00)01195-2. Neurosci Lett. 2000. PMID: 10876067
-
Leptin receptor- and STAT3-immunoreactivities in hypocretin/orexin neurones of the lateral hypothalamus.J Neuroendocrinol. 1999 Aug;11(8):653-63. doi: 10.1046/j.1365-2826.1999.00378.x. J Neuroendocrinol. 1999. PMID: 10447804
-
Hypocretins (orexins): clinical impact of the discovery of a neurotransmitter.Sleep Med Rev. 2005 Aug;9(4):253-68. doi: 10.1016/j.smrv.2005.01.005. Sleep Med Rev. 2005. PMID: 15979356 Review.
-
Regulation of alcohol-seeking by orexin (hypocretin) neurons.Brain Res. 2010 Feb 16;1314:124-9. doi: 10.1016/j.brainres.2009.07.072. Epub 2009 Jul 29. Brain Res. 2010. PMID: 19646424 Review.
Cited by
-
Hypocretin/orexin involvement in reward and reinforcement.Vitam Horm. 2012;89:185-208. doi: 10.1016/B978-0-12-394623-2.00010-X. Vitam Horm. 2012. PMID: 22640614 Free PMC article. Review.
-
Orexin (hypocretin) receptor agonists and antagonists for treatment of sleep disorders. Rationale for development and current status.CNS Drugs. 2013 Feb;27(2):83-90. doi: 10.1007/s40263-012-0036-8. CNS Drugs. 2013. PMID: 23359095 Review.
-
Merkel cells, a new localization of prepro-orexin and orexin receptors.J Anat. 2004 Feb;204(2):117-22. doi: 10.1111/j.1469-7580.2004.00266.x. J Anat. 2004. PMID: 15032918 Free PMC article.
-
SB-334867-A: the first selective orexin-1 receptor antagonist.Br J Pharmacol. 2001 Mar;132(6):1179-82. doi: 10.1038/sj.bjp.0703953. Br J Pharmacol. 2001. PMID: 11250867 Free PMC article.
-
Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates.J Neurosci. 2007 Dec 26;27(52):14239-47. doi: 10.1523/JNEUROSCI.3878-07.2007. J Neurosci. 2007. PMID: 18160631 Free PMC article.
References
-
- Armstrong WE, Warach S, Hatton GI, NcNeill TH. Subnuclei in the rat paraventricular nucleus: a cytoarcitectural, HRP, and immunocytochemical analysis. Neuroscience. 1980;206:317–345. - PubMed
-
- Bittencourt JC, Elias CF. Melanin-concentrating hormone and neuropeptide EI projections from the lateral hypothalamic area and zona incerta to the medial septal nucleus and spinal cord: a study using multiple neuronal tracers. Brain Res. 1998;805:1–19. - PubMed
-
- Bittencourt JC, Presse F, Arias C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. - PubMed
-
- Brown AG. Organization in the spinal cord. The anatomy and physiology of identified neurons, pp 1–238. Springer; New York: 1981.
-
- Cedarbaum JM, Aghajanian GK. Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J Comp Neurol. 1978;178:1–16. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
