Molecular techniques for detection, species differentiation, and phylogenetic analysis of microsporidia
- PMID: 10194459
- PMCID: PMC88917
- DOI: 10.1128/CMR.12.2.243
Molecular techniques for detection, species differentiation, and phylogenetic analysis of microsporidia
Abstract
Microsporidia are obligate intracellular protozoan parasites that infect a broad range of vertebrates and invertebrates. These parasites are now recognized as one of the most common pathogens in human immunodeficiency virus-infected patients. For most patients with infectious diseases, microbiological isolation and identification techniques offer the most rapid and specific determination of the etiologic agent. This is not a suitable procedure for microsporidia, which are obligate intracellular parasites requiring cell culture systems for growth. Therefore, the diagnosis of microsporidiosis currently depends on morphological demonstration of the organisms themselves. Although the diagnosis of microsporidiosis and identification of microsporidia by light microscopy have greatly improved during the last few years, species differentiation by these techniques is usually impossible and transmission electron microscopy may be necessary. Immunfluorescent-staining techniques have been developed for species differentiation of microsporidia, but the antibodies used in these procedures are available only at research laboratories at present. During the last 10 years, the detection of infectious disease agents has begun to include the use of nucleic acid-based technologies. Diagnosis of infection caused by parasitic organisms is the last field of clinical microbiology to incorporate these techniques and molecular techniques (e.g., PCR and hybridization assays) have recently been developed for the detection, species differentiation, and phylogenetic analysis of microsporidia. In this paper we review human microsporidial infections and describe and discuss these newly developed molecular techniques.
Figures
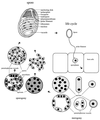


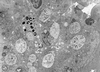


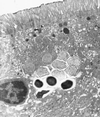

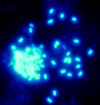

References
-
- Aarons E J, Woodrow D, Hollister W S, Canning E U, Francis N, Gazzard B G. Reversible renal failure caused by a microsporidian infection. AIDS. 1994;8:1119–1121. - PubMed
-
- Albrecht H, Sobottka I, Stellbrinck H J, Greten H. Does choice of Pneumocystis carinii prophylaxis influence the prevalence of Enterocytozoon bieneusi microsporidiosis in AIDS patients? AIDS. 1995;9:302–304. - PubMed
-
- Anwar-Bruni D M, Hogan S E, Schwartz D A, Mel Wilcox C, Bryan R T, Lennox J L. Atovaquone is effective treatment for symptoms of gastrointestinal microsporidiosis in HIV-1-infected patients. AIDS. 1996;10:619–623. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

