Update on diagnosis, management, and prevention of hepatitis B virus infection
- PMID: 10194463
- PMCID: PMC88921
- DOI: 10.1128/CMR.12.2.351
Update on diagnosis, management, and prevention of hepatitis B virus infection
Abstract
Acute and chronic hepatitis B virus (HBV) infection is a leading cause of liver disease worldwide. It is estimated that approximately 350 million people worldwide have chronic HBV infection and that 1 million persons die each year from HBV-related chronic liver disease. In the past decade, significant progress in the understanding of the molecular virology and pathogenesis of HBV infection has been made. In addition, effective treatment modalities have been developed for persons with chronic infection. Worldwide, prevention of HBV transmission has become a high priority. In 1992, the Global Advisory Group to the World Health Organization recommended that all countries integrate hepatitis B vaccine into national immunization programs by 1997. Currently, 80 countries have done so and several others are planning to. Many countries have reported dramatic reductions in the prevalence of chronic HBV infection among children born since the hepatitis B vaccine was introduced into infant immunization schedules. Recent reports from Taiwan indicate a reduction in the incidence of liver cancer among children as a result of widespread hepatitis B vaccination programs.
Figures
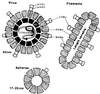
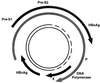



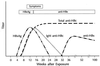




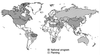
References
-
- Andre F E. Summary of safety and efficacy data on yeast-derived hepatitis B vaccine. Am J Med. 1989;87(Suppl.):39–45. - PubMed
-
- Assateerawatt A, Tanphaichitr V S, Suvatte V, Ingarm L. Immunogenicity and protective efficacy of low dose recombinant DNA hepatitis B vaccine in normal and high-risk neonates. Asian Pac J Allergy Immunol. 1991;9:89–93. - PubMed
-
- Averhoff F, Mahoney F J, Coleman P J, Schatz G, Hurwitz E, Margolis H S. Risk factors for lack of response to hepatitis B vaccine: a randomized trial comparing the immunogenicity of recombinant hepatitis B vaccines in an adult population. Am J Prev Med. 1998;1:73–77. - PubMed
-
- Barin F, Perrin J, Chotard J, Denis F, N’Doye R, Diop Mar I, Chiron J P, Coursaget P. Cross sectional and longtitudinal epidemiology of hepatitis B in Senegal. Prog Med Virol. 1981;27:148–162. - PubMed
-
- Beasley R P. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942–1956. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

