Surfactant protein-A enhances respiratory syncytial virus clearance in vivo
- PMID: 10194474
- PMCID: PMC408263
- DOI: 10.1172/JCI5849
Surfactant protein-A enhances respiratory syncytial virus clearance in vivo
Abstract
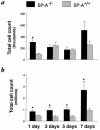
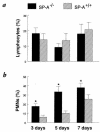
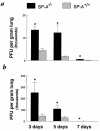
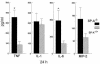
To determine the role of surfactant protein-A(SP-A) in antiviral host defense, mice lacking SP-A (SP-A-/-) were produced by targeted gene inactivation. SP-A-/- and control mice (SP-A+/+) were infected with respiratory syncytial virus (RSV) by intratracheal instillation. Pulmonary infiltration after infection was more severe in SP-A-/- than in SP-A+/+ mice and was associated with increased RSV plaque-forming units in lung homogenates. Pulmonary infiltration with polymorphonuclear leukocytes was greater in the SP-A-/- mice. Levels of proinflammatory cytokines tumor necrosis factor-alpha and interleukin-6 were enhanced in lungs of SP-A-/- mice. After RSV infection, superoxide and hydrogen peroxide generation was deficient in macrophages from SP-A-/- mice, demonstrating a critical role of SP-A in oxidant production associated with RSV infection. Coadministration of RSV with exogenous SP-A reduced viral titers and inflammatory cells in the lung of SP-A-/- mice. These findings demonstrate that SP-A plays an important host defense role against RSV in vivo.
Figures


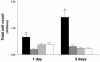
References
-
- Thiel S, Reid K. Structures and functions associated with the group of mammalian lectins containing collagen-like sequences. FEBS Lett. 1989;250:78–84. - PubMed
-
- Sastry K, Ezekowitz RA. Collectins: pattern recognition molecules involved in first line host defense. Curr Opin Immunol. 1993;5:59–66. - PubMed
-
- Super M, Thiel S, Lu J, Levinsky RJ, Turner MW. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989;2:1236–1239. - PubMed
-
- Friis-Christiansen P, et al. In vivo and in vitro antibacterial activity of conglutinin, a mammalian plasma lectin. Scand J Immunol. 1990;31:453–460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

