A phosphotransferase that generates phosphatidylinositol 4-phosphate (PtdIns-4-P) from phosphatidylinositol and lipid A in Rhizobium leguminosarum. A membrane-bound enzyme linking lipid a and ptdins-4-p biosynthesis
- PMID: 10196199
- PMCID: PMC2548417
- DOI: 10.1074/jbc.274.16.11139
A phosphotransferase that generates phosphatidylinositol 4-phosphate (PtdIns-4-P) from phosphatidylinositol and lipid A in Rhizobium leguminosarum. A membrane-bound enzyme linking lipid a and ptdins-4-p biosynthesis
Abstract
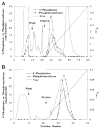
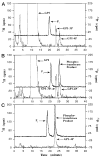
Membranes of Rhizobium leguminosarum contain a 3-deoxy-D-manno-octulosonic acid (Kdo)-activated lipid A 4'-phosphatase required for generating the unusual phosphate-deficient lipid A found in this organism. The enzyme has been solubilized with Triton X-100 and purified 80-fold. As shown by co-purification and thermal inactivation studies, the 4'-phosphatase catalyzes not only the hydrolysis of (Kdo)2-[4'-32P]lipid IVA but also the transfer the 4'-phosphate of Kdo2-[4'-32P]lipid IVA to the inositol headgroup of phosphatidylinositol (PtdIns) to generate PtdIns-4-P. Like the 4'-phosphatase, the phosphotransferase activity is not present in Escherichia coli, Rhizobium meliloti, or the nodulation-defective mutant 24AR of R. leguminosarum. The specific activity for the phosphotransferase reaction is about 2 times higher than that of the 4'-phosphatase. The phosphotransferase assay conditions are similar to those used for PtdIns kinases, except that ATP and Mg2+ are omitted. The apparent Km for PtdIns is approximately 500 microM versus 20-100 microM for most PtdIns kinases, but the phosphotransferase specific activity in crude cell extracts is higher than that of most PtdIns kinases. The phosphotransferase is absolutely specific for the 4-position of PtdIns and is highly selective for PtdIns as the acceptor. The 4'-phosphatase/phosphotransferase can be eluted from heparin- or Cibacron blue-agarose with PtdIns. A phosphoenzyme intermediate may account for the dual function of this enzyme, since a single 32P-labeled protein species (Mr approximately 68,000) can be trapped and visualized by SDS gel electrophoresis of enzyme preparations incubated with Kdo2-[4'-32P]lipid IVA. Although PtdIns is not detected in cultures of R. leguminosarum/etli (CE3), PtdIns may be synthesized during nodulation or supplied by plant membranes, given that soybean PtdIns is an excellent phosphate acceptor. A bacterial enzyme for generating PtdIns-4-P and a direct link between lipid A and PtdIns-4-P biosynthesis have not been reported previously.
Figures










References
-
- Raetz CRH. Annu Rev Biochem. 1990;59:129–170. - PubMed
-
- Raetz CRH. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. 2. Neidhardt FC, editor. Vol. 1. American Society for Microbiology; Washington, D. C: 1996. pp. 1035–1063.
-
- Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zähringer U, Seydel U, Di Padova F, Schreier M, Brade H. FASEB J. 1994;8:217–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

