Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies
- PMID: 10196297
- PMCID: PMC104180
- DOI: 10.1128/JVI.73.5.4009-4018.1999
Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies
Abstract
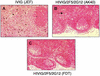
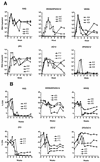
The role of antibody in protection against human immunodeficiency virus (HIV-1) has been difficult to study in animal models because most primary HIV-1 strains do not infect nonhuman primates. Using a chimeric simian/human immunodeficiency virus (SHIV) based on the envelope of a primary isolate (HIV-89.6), we performed passive-transfer experiments in rhesus macaques to study the role of anti-envelope antibodies in protection. Based on prior in vitro data showing neutralization synergy by antibody combinations, we evaluated HIV immune globulin (HIVIG), and human monoclonal antibodies (MAbs) 2F5 and 2G12 given alone, compared with the double combination 2F5/2G12 and the triple combination HIVIG/2F5/2G12. Antibodies were administered 24 h prior to intravenous challenge with the pathogenic SHIV-89.6PD. Six control monkeys displayed high plasma viremia, rapid CD4(+)-cell decline, and clinical AIDS within 14 weeks. Of six animals given HIVIG/2F5/2G12, three were completely protected; the remaining three animals became SHIV infected but displayed reduced plasma viremia and near normal CD4(+)-cell counts. One of three monkeys given 2F5/2G12 exhibited only transient evidence of infection; the other two had marked reductions in viral load. All monkeys that received HIVIG, 2F5, or 2G12 alone became infected and developed high-level plasma viremia. However, compared to controls, monkeys that received HIVIG or MAb 2G12 displayed a less profound drop in CD4(+) T cells and a more benign clinical course. These data indicate a general correlation between in vitro neutralization and protection and suggest that a vaccine that elicits neutralizing antibody should have a protective effect against HIV-1 infection or disease.
Figures
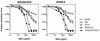


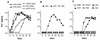
References
-
- Bahmanyar M, Fayaz A, Nour-Salehi S, Mohammadi M, Koprowski H. Successful protection of humans exposed to rabies infection: postexposure treatment with the new human diploid cell rabies vaccine and antirabies serum. JAMA. 1976;236:2751–2754. - PubMed
-
- Beasley R P, Hwang L Y, Steven C E. Efficacy of hepatitis B immune globulin for prevention of perinatal transmission of the hepatitis B virus carrier state: final report of a randomized double-blind, placebo-controlled trial. Hepatology. 1983;3:135–141. - PubMed
-
- Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L J, Mackay C R, Larosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

