In vitro infection of human peripheral blood mononuclear cells by GB virus C/Hepatitis G virus
- PMID: 10196301
- PMCID: PMC104184
- DOI: 10.1128/JVI.73.5.4052-4061.1999
In vitro infection of human peripheral blood mononuclear cells by GB virus C/Hepatitis G virus
Abstract
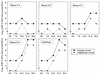
GB virus C (GBV-C), also known as hepatitis G virus, is a recently discovered flavivirus-like RNA agent with unclear pathogenic implications. To investigate whether human peripheral blood mononuclear cells (PBMC) are susceptible to in vitro GBV-C infection, we have incubated PBMC from four healthy blood donors with a human GBV-C RNA-positive serum. By means of (i) strand-specific reverse transcription-PCR, cloning, and sequencing; (ii) sucrose ultracentrifugation and RNase sensitivity assays; (iii) fluorescent in situ hybridization; and (iv) Western blot analysis, it has been demonstrated that GBV-C is able to infect in vitro cells and replicate for as long as 30 days under the conditions developed in our cell culture system. The concentration of GBV-C RNA increased during the second and third weeks of culture. The titers of the genomic strand were 10 times higher than the titers of the antigenomic strand. In addition, the same predominant GBV-C sequence was found in all PBMC cultures and in the in vivo-GBV-C-infected PBMC isolated from the donor of the inoculum. GBV-C-specific fluorescent in situ hybridization signals were confined to the cytoplasm of cells at different times during the culture period. Finally, evidence obtained by sucrose ultracentrifugation, RNase sensitivity assays, and Western blot analysis of the culture supernatants suggests that viral particles are released from in vitro-GBV-C-infected PBMC. In conclusion, our study has demonstrated, for the first time, GBV-C replication in human lymphoid cells under experimental in vitro infection conditions.
Figures







References
-
- Aikawa T, Sugay Y, Okamoto H. Hepatitis G infection in drug abusers with chronic hepatitis C. N Engl J Med. 1996;334:195. - PubMed
-
- Alter H J, Nakatsuyi Y, Melpolder J, Wages J, Wesley R, Shih J W K, Kim J P. The incidence of transfusion-associated hepatitis G virus infection and its relation to liver disease. N Engl J Med. 1997;336:747–754. - PubMed
-
- Berenguer M, Terrault N A, Piatak M, Yun A, Kim J P, Lau J Y N, Lake J R, Roberts J R, Ascher N, Ferrell L, Wright T. Hepatitis G virus infection in patients with hepatitis C virus infection undergoing liver transplantation. Gastroenterology. 1996;111:1569–1575. - PubMed
-
- Bukh J, Kim J P, Govindarajan S, Apgar C L, Foung S K H, Wages J, Jr, Yun A J, Shapiro M, Emerson S U, Purcell R H. Experimental infection of chimpanzees with hepatitis G virus and genetic analysis of the virus. J Infect Dis. 1998;177:855–862. - PubMed
-
- Chomzynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

