Mutational analysis of glycosylation, membrane translocation, and cell surface expression of the hepatitis E virus ORF2 protein
- PMID: 10196303
- PMCID: PMC104186
- DOI: 10.1128/JVI.73.5.4074-4082.1999
Mutational analysis of glycosylation, membrane translocation, and cell surface expression of the hepatitis E virus ORF2 protein
Abstract
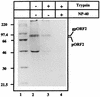
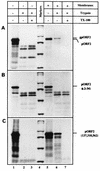
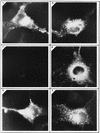
Hepatitis E virus (HEV) is the etiological agent for viral hepatitis type E, which is a major problem in the developing world. Because HEV cannot be cultured in vitro, very little information exists on the mechanisms of HEV gene expression and genome replication. HEV is a positive-strand RNA virus with three potential open reading frames (ORFs), one of which (ORF2) is postulated to encode the major viral capsid protein (pORF2). We earlier showed (S. Jameel, M. Zafrullah, M. H. Ozdener, and S. K. Panda, J. Virol. 70:207-216, 1996) pORF2 to be a approximately 88-kDa glycoprotein, carrying N-linked glycans and a potential endoplasmic reticulum (ER)-directing signal at its N terminus. Treatment with the drugs brefeldin A and monensin suggest that the protein may accumulate within the ER. Based on mutational analysis, we demonstrate Asn-310 to be the major site of N-glycan addition. In COS-1 cell expression and in vitro translation experiments, we confirm the ER-translocating nature of the pORF2 N-terminal hydrophobic sequence and show that the protein is cotranslationally, but not posttranslationally, translocated across the ER membrane. Earlier, we had also demonstrated cell surface localization of a fraction of the COS-1 cell-expressed pORF2. Using glycosylation- and translocation-defective mutants of pORF2, we now show that while transit of pORF2 into the ER is necessary for its cell surface expression, glycosylation of the protein is not required for such localization. These results may offer clues to the mechanisms of gene expression and capsid assembly in HEV.
Figures

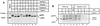


References
-
- Bi S L, Purdy M A, McCaustland K A, Margolis H S, Bradley D W. The sequence of hepatitis E virus isolated directly from a single source during an outbreak in China. Virus Res. 1993;28:233–247. - PubMed
-
- Bradley D W. Enterically-transmitted non-A, non-B hepatitis. Br Med Bull. 1990;46:442–461. - PubMed
-
- Fielder K, Simmons K. The role of N-glycans in the secretory pathway. Cell. 1995;81:309–312. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

