Live-cell analysis of a green fluorescent protein-tagged herpes simplex virus infection
- PMID: 10196307
- PMCID: PMC104190
- DOI: 10.1128/JVI.73.5.4110-4119.1999
Live-cell analysis of a green fluorescent protein-tagged herpes simplex virus infection
Abstract
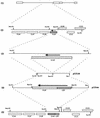
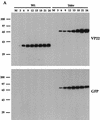
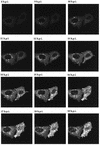
Many stages of the herpes simplex virus maturation pathway have not yet been defined. In particular, little is known about the assembly of the virion tegument compartment and its subsequent incorporation into maturing virus particles. Here we describe the construction of a herpes simplex virus type 1 (HSV-1) recombinant in which we have replaced the gene encoding a major tegument protein, VP22, with a gene expressing a green fluorescent protein (GFP)-VP22 fusion protein (GFP-22). We show that this virus has growth properties identical to those of the parental virus and that newly synthesized GFP-22 is detectable in live cells as early as 3 h postinfection. Moreover, we show that GFP-22 is incorporated into the HSV-1 virion as efficiently as VP22, resulting in particles which are visible by fluorescence microscopy. Consequently, we have used time lapse confocal microscopy to monitor GFP-22 in live-cell infection, and we present time lapse animations of GFP-22 localization throughout the virus life cycle. These animations demonstrate that GFP-22 is present in a diffuse cytoplasmic location when it is initially expressed but evolves into particulate material which travels through an exclusively cytoplasmic pathway to the cell periphery. In this way, we have for the first time visualized the trafficking of a herpesvirus structural component within live, infected cells.
Figures






References
-
- Dargin D. The structure and assembly of herpes viruses. In: Harris J R, Horne R W, editors. Electron microscopy of proteins. 5. Virus structure. London, United Kingdom: Academic Press; 1986. pp. 359–437.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

