Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease
- PMID: 10196314
- PMCID: PMC104197
- DOI: 10.1128/JVI.73.5.4181-4187.1999
Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease
Abstract
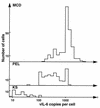
Human herpesvirus 8 (HHV-8) infection has been implicated in the etiology of Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman's disease (MCD), three diseases that frequently develop in immunocompromised, human immunodeficiency virus-positive individuals. One hypothesis that would account for different pathological manifestations of infection by the same virus is that viral genes are differentially expressed in heterogeneous cell types. To test this hypothesis, we analyzed the localization and levels of expression of two viral genes expressed in latent and lytic infections and the viral homologue of interleukin-6 (vIL-6). We show that PEL parallels KS in the pattern of latent and lytic cycle viral gene expression but that the predominant infected cell type is a B cell. We also show that MCD differs from KS not only in the infected cell type (B-cell and T-cell lineage) but also in the pattern of viral gene expression. Only a few cells in the lesion are infected and all of these cells express lytic-cycle genes. Of possibly greater significance is the fact that in a comparison of KS, PEL, and MCD, we found dramatic differences in the levels of expression of vIL-6. Interleukin-6 is a B-cell growth and differentiation factor whose altered expression has been linked to plasma cell abnormalities, as well as myeloid and lymphoid malignancies. Our findings support the hypothesis that HHV-8 plays an important role in the pathogenesis of PEL and MCD, in which vIL-6 acts as an autocrine or paracrine factor in the lymphoproliferative processes common to both.
Figures



References
-
- Ansari M Q, Dawson D B, Nador R, Rutherford C, Schneider N R, Latimer M J, Ricker L, Knowles D M, McKenna R W. Primary body cavity-based AIDS-related lymphomas. Am J Clin Pathol. 1996;105:141–143. - PubMed
-
- Arcone R, Pucci P, Zappacosta F, Fontaine V, Malorni A, Marino G, Ciliberto G. Single-step purification and structural characterization of human interleukin-6 produced in Escherichia coli from a T7 RNA polymerase expression vector. Eur J Biochem. 1991;198:541–547. - PubMed
-
- Burger R, Wendler J, Antoni K, Helm G, Kalden J R, Gramatzki M. Interleukin-6 production in B-cell neoplasias and Castleman’s disease: evidence for an additional paracrine loop. Ann Hematol. 1994;69:25–31. - PubMed
-
- Castleman B, Iverson L, Menendez V P. Localized mediastinal lymph-node hyperplasia resembling thymoma. Cancer. 1956;9:822–830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

