The amino-terminal region of Vpr from human immunodeficiency virus type 1 forms ion channels and kills neurons
- PMID: 10196319
- PMCID: PMC104202
- DOI: 10.1128/JVI.73.5.4230-4238.1999
The amino-terminal region of Vpr from human immunodeficiency virus type 1 forms ion channels and kills neurons
Abstract
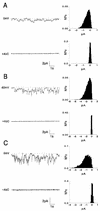
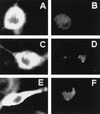
We have previously reported that the accessory protein Vpr from human immunodeficiency virus type 1 forms cation-selective ion channels in planar lipid bilayers and is able to depolarize intact cultured neurons by causing an inward sodium current, resulting in cell death. In this study, we used site-directed mutagenesis and synthetic peptides to identify the structural regions responsible for the above functions. Mutations in the N-terminal region of Vpr were found to affect channel activity, whereas this activity was not affected by mutations in the hydrophobic region of Vpr (amino acids 53 to 71). Analysis of mutants containing changes in the basic C terminus confirmed previous results that this region, although not necessary for ion channel function, was responsible for the observed rectification of wild-type Vpr currents. A peptide comprising the first 40 N-terminal amino acids of Vpr (N40) was found to be sufficient to form ion channels similar to those caused by wild-type Vpr in planar lipid bilayers. Furthermore, N40 was able to cause depolarization of the plasmalemma and cell death in cultured hippocampal neurons with a time course similar to that seen with wild-type Vpr, supporting the idea that this region is responsible for Vpr ion channel function and cytotoxic effects. Since Vpr is found in the serum and cerebrospinal fluids of AIDS patients, these results may have significance for AIDS pathology.
Figures




Similar articles
-
Extracellular HIV-1 virus protein R causes a large inward current and cell death in cultured hippocampal neurons: implications for AIDS pathology.Proc Natl Acad Sci U S A. 1998 Apr 14;95(8):4595-600. doi: 10.1073/pnas.95.8.4595. Proc Natl Acad Sci U S A. 1998. PMID: 9539783 Free PMC article.
-
Vpr protein of human immunodeficiency virus type 1 forms cation-selective channels in planar lipid bilayers.Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):111-5. doi: 10.1073/pnas.93.1.111. Proc Natl Acad Sci U S A. 1996. PMID: 8552585 Free PMC article.
-
The carboxy-terminal domain is essential for stability and not for virion incorporation of HIV-1 Vpr into virus particles.Virology. 1995 Dec 20;214(2):647-52. doi: 10.1006/viro.1995.0079. Virology. 1995. PMID: 8553570
-
Partner molecules of accessory protein Vpr of the human immunodeficiency virus type 1.DNA Cell Biol. 2004 Apr;23(4):193-205. doi: 10.1089/104454904773819789. DNA Cell Biol. 2004. PMID: 15142377 Review.
-
HIV-1 Vpr: genetic diversity and functional features from the perspective of structure.DNA Cell Biol. 2004 Apr;23(4):207-22. doi: 10.1089/104454904773819798. DNA Cell Biol. 2004. PMID: 15142378 Review.
Cited by
-
Interactions between human immunodeficiency virus (HIV)-1 Vpr expression and innate immunity influence neurovirulence.Retrovirology. 2011 Jun 6;8:44. doi: 10.1186/1742-4690-8-44. Retrovirology. 2011. PMID: 21645334 Free PMC article.
-
Extracellular human immunodeficiency virus type 1 viral protein R causes reductions in astrocytic ATP and glutathione levels compromising the antioxidant reservoir.Virus Res. 2012 Aug;167(2):358-69. doi: 10.1016/j.virusres.2012.06.002. Epub 2012 Jun 9. Virus Res. 2012. PMID: 22691542 Free PMC article.
-
Expression, purification, and activities of full-length and truncated versions of the integral membrane protein Vpu from HIV-1.Protein Sci. 2002 Mar;11(3):546-57. doi: 10.1110/ps.37302. Protein Sci. 2002. PMID: 11847278 Free PMC article.
-
Effect of extracellular HIV-1 Vpr protein in vitro.J Neurovirol. 2001 Apr;7(2):183-5. doi: 10.1080/13550280152058843. J Neurovirol. 2001. PMID: 11517391 No abstract available.
-
Critical implication of the (70-96) domain of human immunodeficiency virus type 1 Vpr protein in apoptosis of primary rat cortical and striatal neurons.J Neurovirol. 2005 Dec;11(6):489-502. doi: 10.1080/13550280500384941. J Neurovirol. 2005. PMID: 16338743
References
-
- Agawa Y, Lee S, Ono S, Aoyagi H, Taniguchi T, Anzai K, Kirino Y. Interaction with phospholipid bilayers, ion channel formation, and antimicrobial activity of basic amphipathic alpha-helical model peptides of various chain lengths. J Biol Chem. 1991;266:20218–20222. - PubMed
-
- Arunagiri C, Macreadie I G, Hewish D, Azad A A. A C-terminal domain of HIV-1 accessory protein Vpr is involved in penetration, mitochondrial dysfunction and apoptosis of human CD4+ lymphocytes. Apoptosis. 1997;2:69–76. - PubMed
-
- Balliet J W, Kolson D L, Eiger G, Kim F M, McGann K A, Srinivasan A, Collman R. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology. 1994;200:623–631. - PubMed
-
- Berry E A, Hinkle P C. Measurement of the electrochemical proton gradient in submitochondrial particles. J Biol Chem. 1983;258:1474–1486. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

