kdgREcc negatively regulates genes for pectinases, cellulase, protease, HarpinEcc, and a global RNA regulator in Erwinia carotovora subsp. carotovora
- PMID: 10198003
- PMCID: PMC93665
- DOI: 10.1128/JB.181.8.2411-2421.1999
kdgREcc negatively regulates genes for pectinases, cellulase, protease, HarpinEcc, and a global RNA regulator in Erwinia carotovora subsp. carotovora
Abstract
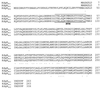
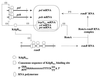
Erwinia carotovora subsp. carotovora produces extracellular pectate lyase (Pel), polygalacturonase (Peh), cellulase (Cel), and protease (Prt). The concerted actions of these enzymes largely determine the virulence of this plant-pathogenic bacterium. E. carotovora subsp. carotovora also produces HarpinEcc, the elicitor of the hypersensitive reaction. We document here that KdgREcc (Kdg, 2-keto-3-deoxygluconate; KdgR, general repressor of genes involved in pectin and galacturonate catabolism), a homolog of the E. chrysanthemi repressor, KdgREch and the Escherichia coli repressor, KdgREco, negatively controls not only the pectinases, Pel and Peh, but also Cel, Prt, and HarpinEcc production in E. carotovora subsp. carotovora. The levels of pel-1, peh-1, celV, and hrpNEcc transcripts are markedly affected by KdgREcc. The KdgREcc- mutant is more virulent than the KdgREcc+ parent. Thus, our data for the first time establish a global regulatory role for KdgREcc in E. carotovora subsp. carotovora. Another novel observation is the negative effect of KdgREcc on the transcription of rsmB (previously aepH), which specifies an RNA regulator controlling exoenzyme and HarpinEcc production. The levels of rsmB RNA are higher in the KdgREcc- mutant than in the KdgREcc+ parent. Moreover, by DNase I protection assays we determined that purified KdgREcc protected three 25-bp regions within the transcriptional unit of rsmB. Alignment of the protected sequences revealed the 21-mer consensus sequence of the KdgREcc-binding site as 5'-G/AA/TA/TGAAA[N6]TTTCAG/TG/TA-3'. Two such KdgREcc-binding sites occur in rsmB DNA in a close proximity to each other within nucleotides +79 and +139 and the third KdgREcc-binding site within nucleotides +207 and +231. Analysis of lacZ transcriptional fusions shows that the KdgR-binding sites negatively affect the expression of rsmB. KdgREcc also binds the operator DNAs of pel-1 and peh-1 genes and represses expression of a pel1-lacZ and a peh1-lacZ transcriptional fusions. We conclude that KdgREcc affects extracellular enzyme production by two ways: (i) directly, by inhibiting the transcription of exoenzyme genes; and (ii) indirectly, by preventing the production of a global RNA regulator. Our findings support the idea that KdgREcc affects transcription by promoter occlusion, i.e., preventing the initiation of transcription, and by a roadblock mechanism, i.e., by affecting the elongation of transcription.
Figures






References
-
- Aiba H, Adhya S, de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981;256:11905–11910. - PubMed
-
- Barras F, van Gijsegem F, Chatterjee A K. Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annu Rev Phytopathol. 1994;32:201–234.
-
- Bauer D W, Wei Z-M, Beer S V, Collmer A. Erwinia chrysanthemi harpinEch: an elicitor of the hypersensitive response that contributes to soft-rot pathogenesis. Mol Plant-Microbe Interact. 1995;8:484–491. - PubMed
-
- Chatterjee A, Cui Y, Liu Y, Dumenyo C K, Chatterjee A K. Inactivation of rsmA leads to overproduction of extracellular pectinases, cellulases, and proteases in Erwinia carotovora subsp. carotovora in the absence of the starvation/cell density sensing signal, N-(3-oxohexanoyl)-l-homoserine lactone. Appl Environ Microbiol. 1995;61:1959–1967. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

