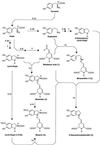
The decisive step in betaxanthin biosynthesis is a spontaneous reaction1
- PMID: 10198080
- PMCID: PMC32006
- DOI: 10.1104/pp.119.4.1217
The decisive step in betaxanthin biosynthesis is a spontaneous reaction1
Abstract
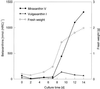
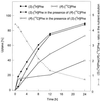
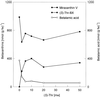
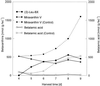
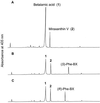
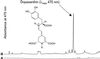
Experiments were performed to confirm that the aldimine bond formation is a spontaneous reaction, because attempts to find an enzyme catalyzing the last decisive step in betaxanthin biosynthesis, the aldimine formation, failed. Feeding different amino acids to betalain-forming hairy root cultures of yellow beet (Beta vulgaris L. subsp. vulgaris "Golden Beet") showed that all amino acids (S- and R-forms) led to the corresponding betaxanthins. We observed neither an amino acid specificity nor a stereoselectivity in this process. In addition, increasing the endogenous phenylalanine (Phe) level by feeding the Phe ammonia-lyase inhibitor 2-aminoindan 2-phosphonic acid yielded the Phe-derived betaxanthin. Feeding amino acids or 2-aminoindan 2-phosphonic acid to hypocotyls of fodder beet (B. vulgaris L. subsp. vulgaris "Altamo") plants led to the same results. Furthermore, feeding cyclo-3-(3,4-dihydroxyphenyl)-alanine (cyclo-Dopa) to these hypocotyls resulted in betanidin formation, indicating that the decisive step in betacyanin formation proceeds spontaneously. Finally, feeding betalamic acid to broad bean (Vicia faba L.) seedlings, which are known to accumulate high levels of Dopa but do not synthesize betaxanthins, resulted in the formation of dopaxanthin. These results indicate that the condensation of betalamic acid with amino acids (possibly including cyclo-Dopa or amines) in planta is a spontaneous, not an enzyme-catalyzed reaction.
Figures



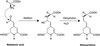
References
-
- Adachi T, Nakatsukasa M, Asaka Y, Uta T. Genetic analysis and some properties of flower color mutants found in the progenies of x-ray irradiated Portulaca sp. “Jewel.”. Jpn J Breed. 1985;35:183–192.
-
- Bianco-Colomas J. Qualitative and quantitative aspects of betalains biosynthesis in Amaranthus caudatus L. var. pendula seedlings. Planta. 1980;149:176–180. - PubMed
-
- Böhm H, Hermersdörfer H, Schönfeld A. Betaxanthinbildende hairy-root-kulturen von Beta vulgaris var. lutea. Vorträge für Pflanzenzüchtung. 1991;21:105–108.
-
- Bokern M, Heuer S, Wray V, Witte L, Macek T, Vanek T, Strack D. Ferulic acid conjugates and betacyanins from cell cultures of Beta vulgaris. Phytochemistry. 1991;30:3261–3265.
-
- De-Eknamkul W, Ounaroon A, Tanahashi T, Kutchan TM, Zenk MH. Phytochemistry. 1997;45:477–484.
LinkOut - more resources
Full Text Sources

