Identification, separation, and characterization of acyl-coenzyme A dehydrogenases involved in mitochondrial beta-oxidation in higher plants
- PMID: 10198089
- PMCID: PMC32015
- DOI: 10.1104/pp.119.4.1305
Identification, separation, and characterization of acyl-coenzyme A dehydrogenases involved in mitochondrial beta-oxidation in higher plants
Abstract
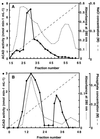
The existence in higher plants of an additional beta-oxidation system in mitochondria, besides the well-characterized peroxisomal system, is often considered controversial. Unequivocal demonstration of beta-oxidation activity in mitochondria should rely on identification of the enzymes specific to mitochondrial beta-oxidation. Acyl-coenzyme A dehydrogenase (ACAD) (EC 1.3.99.2,3) activity was detected in purified mitochondria from maize (Zea mays L.) root tips and from embryonic axes of early-germinating sunflower (Helianthus annuus L.) seeds, using as the enzyme assay the reduction of 2,6-dichlorophenolindophenol, with phenazine methosulfate as the intermediate electron carrier. Subcellular fractionation showed that this ACAD activity was associated with mitochondrial fractions. Comparison of ACAD activity in mitochondria and acyl-coenzyme A oxidase activity in peroxisomes showed differences of substrate specificities. Embryonic axes of sunflower seeds were used as starting material for the purification of ACADs. Two distinct ACADs, with medium-chain and long-chain substrate specificities, respectively, were separated by their chromatographic behavior, which was similar to that of mammalian ACADs. The characterization of these ACADs is discussed in relation to the identification of expressed sequenced tags corresponding to ACADs in cDNA sequence analysis projects and with the potential roles of mitochondrial beta-oxidation in higher plants.
Figures


References
-
- Aalen N, Steen IH, Birkeland NK, Lien T. Purification and properties of an extremely thermostable NADP+-specific glutamate dehydrogenase from Archaeoglobus fulgidus. Arch Microbiol. 1997;168:536–539. - PubMed
-
- Aebi HE. Catalase. In: Bergmeyer HU, Bergmeyer J, Grassl M, editors. Methods of Enzymatic Analysis, Vol 3. Weinheim, Germany: VCH; 1987. pp. 273–286.
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman D. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Aoyama T, Souri M, Kamijo T, Ushikubo S, Hashimoto T. Peroxisomal acyl-coenzyme A oxidase is a rate-limiting enzyme in a very-long-chain fatty acid β-oxidation system. Biochem Biophys Res Commun. 1994a;201:1541–1547. - PubMed
-
- Aoyama T, Ueno I, Kamijo T, Hashimoto T. Rat very-long-chain acyl-CoA dehydrogenase, a novel mitochondrial acyl-CoA dehydrogenase gene product, is a rate-limiting enzyme in long-chain fatty acid β-oxidation system. J Biol Chem. 1994b;269:19088–19094. - PubMed
LinkOut - more resources
Full Text Sources

