Cyanide-resistant, ATP-synthesis-sustained, and uncoupling-protein-sustained respiration during postharvest ripening of tomato fruit
- PMID: 10198091
- PMCID: PMC32017
- DOI: 10.1104/pp.119.4.1323
Cyanide-resistant, ATP-synthesis-sustained, and uncoupling-protein-sustained respiration during postharvest ripening of tomato fruit
Abstract
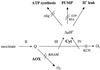
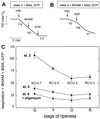
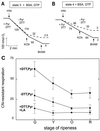
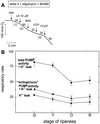
Tomato (Lycopersicon esculentum) mitochondria contain both alternative oxidase (AOX) and uncoupling protein as energy-dissipating systems that can decrease the efficiency of oxidative phosphorylation. We followed the cyanide (CN)-resistant, ATP-synthesis-sustained, and uncoupling-protein-sustained respiration of isolated mitochondria, as well as the immunologically detectable levels of uncoupling protein and AOX, during tomato fruit ripening from the mature green stage to the red stage. The AOX protein level and CN-resistant respiration of isolated mitochondria decreased with ripening from the green to the red stage. The ATP-synthesis-sustained respiration followed the same behavior. In contrast, the level of uncoupling protein and the total uncoupling-protein-sustained respiration of isolated mitochondria decreased from only the yellow stage on. We observed an acute inhibition of the CN-resistant respiration by linoleic acid in the micromolar range. These results suggest that the two energy-dissipating systems could have different roles during the ripening process.
Figures

References
-
- Andrews J. The climacteric respiration rise in attached and detached tomato fruit. Posharvest Biol Technol. 1995;6:287–292.
-
- Cruz-Hernandez A, Gomez-Lim MA. Alternative oxidase from mango (Mangifera indica L.) is differentially regulated during fruit ripening. Planta. 1995;197:569–576. - PubMed
-
- Day DA, Arron GP, Laties GG (1980) Nature and control of respiratory pathways in plants: the interaction of CN-resistant respiration with CN-sensitive pathway. In DD Davies, ed, The Biochemistry of Plants, Vol 4. Academic Press, New York, pp 197–241
-
- Flores H, Chin CK. Effects of KCN and salicylhydroxamic acid on the respiration and growth of excised tomato roots. Plant Sci Lett. 1980;17:237–243.
LinkOut - more resources
Full Text Sources

