Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane
- PMID: 10198097
- PMCID: PMC32023
- DOI: 10.1104/pp.119.4.1379
Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane
Abstract
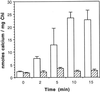
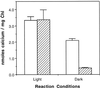
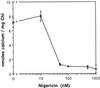
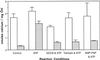
To assess the availability of Ca2+ in the lumen of the thylakoid membrane that is required to support the assembly of the oxygen-evolving complex of photosystem II, we have investigated the mechanism of 45Ca2+ transport into the lumen of pea (Pisum sativum) thylakoid membranes using silicone-oil centrifugation. Trans-thylakoid Ca2+ transport is dependent on light or, in the dark, on exogenously added ATP. Both light and ATP hydrolysis are coupled to Ca2+ transport through the formation of a transthylakoid pH gradient. The H+-transporting ionophores nigericin/K+ and carbonyl cyanide 3-chlorophenylhydrazone inhibit the transport of Ca2+. Thylakoid membranes are capable of accumulating up to 30 nmol Ca2+ mg-1 chlorophyll from external concentrations of 15 μM over the course of a 15-min reaction. These results are consistent with the presence of an active Ca2+/H+ antiport in the thylakoid membrane. Ca2+ transport across the thylakoid membrane has significant implications for chloroplast and plant Ca2+ homeostasis. We propose a model of chloroplast Ca2+ regulation whereby the activity of the Ca2+/H+ antiporter facilitates the light-dependent uptake of Ca2+ by chloroplasts and reduces stromal Ca2+ levels.
Figures


References
-
- Barber J, Mills JB, Nicolson J. Studies with cation specific ionophores show that within the intact chloroplast Mg2+ acts as the main exchange cation for H+ pumping. FEBS Lett. 1974;49:106–110. - PubMed
-
- Becker DW, Callahan FE, Cheniae GM. Photoactivation of NH2OH-treated leaves: reassembly of released extrinsic PSII polypeptides and relegation of Mn into the polynuclear Mn catalyst of water oxidation. FEBS Lett. 1985;192:209–214.
-
- Blackford S, Rea PA, Sanders D. Voltage sensitivity of H+/Ca2+ antiport in higher plant tonoplast suggests a role in vacuolar calcium accumulation. J Biol Chem. 1990;265:9617–9620. - PubMed
-
- Broussac A, Zimmermann J-L, Rutherford AW, Lavergne J. Histidine oxidation in the oxygen-evolving photosystem II enzyme. Nature. 1990;347:303–306.
LinkOut - more resources
Full Text Sources
Miscellaneous

