Protective function of chloroplast 2-cysteine peroxiredoxin in photosynthesis. Evidence from transgenic Arabidopsis
- PMID: 10198100
- PMCID: PMC32026
- DOI: 10.1104/pp.119.4.1407
Protective function of chloroplast 2-cysteine peroxiredoxin in photosynthesis. Evidence from transgenic Arabidopsis
Abstract
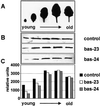
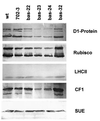
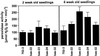
2-Cysteine peroxiredoxins (2-CPs) constitute a ubiquitous group of peroxidases that reduce cell-toxic alkyl hydroperoxides to their corresponding alcohols. Recently, we cloned 2-CP cDNAs from plants and characterized them as chloroplast proteins. To elucidate the physiological function of the 2-CP in plant metabolism, we generated antisense mutants in Arabidopsis. In the mutant lines a 2-CP deficiency developed during early leaf and plant development and eventually the protein accumulated to wild-type levels. In young mutants with reduced amounts of 2-CP, photosynthesis was impaired and the levels of D1 protein, the light-harvesting protein complex associated with photosystem II, chloroplast ATP synthase, and ribulose-1,5-bisphosphate carboxylase/oxygenase were decreased. Photoinhibition was particularly pronounced after the application of the protein synthesis inhibitor, lincomycin. We concluded that the photosynthetic machinery needs high levels of 2-CP during leaf development to protect it from oxidative damage and that the damage is reduced by the accumulation of 2-CP protein, by the de novo synthesis and replacement of damaged proteins, and by the induction of other antioxidant defenses in 2-CP mutants.
Figures




References
-
- Baier M, Bilger W, Wolf R, Dietz K-J. Photosynthesis in the basal growing zone of barley leaves. Photosynth Res. 1996;49:169–181. - PubMed
-
- Baier M, Dietz K-J. Primary structure and expression of plant homologues of animal and fungal thioredoxin-dependent peroxide reductases and bacterial alkyl hydroperoxide reductases. Plant Mol Biol. 1996a;31:553–564. - PubMed
-
- Baier M, Dietz K-J. 2-Cys peroxiredoxin bas1 from Arabidopsis thaliana (accession no. X94218) (PGR 96-031) Plant Physiol. 1996b;111:651.
-
- Baier M, Dietz K-J (1996c) The 2-Cys peroxiredoxin BAS1: insight in a new family of plant peroxidases. In C Obinger, U Burner, R Ebermann, C Penel, H Greppin, eds, Plant Peroxidases: Biochemistry and Physiology. University of Geneva, Switzerland, pp 204–209
-
- Baier M, Dietz K-J. The plant 2-Cys peroxiredoxin BAS1 is a nuclear-encoded chloroplast protein: its expressional regulation, phylogenetic origin, and implications for its specific physiological function in plants. Plant J. 1997;12:179–190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

