New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway
- PMID: 10200246
- PMCID: PMC33561
- DOI: 10.1073/pnas.96.8.4240
New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway
Abstract
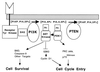
The most recently discovered PTEN tumor suppressor gene has been found to be defective in a large number of human cancers. In addition, germ-line mutations in PTEN result in the dominantly inherited disease Cowden syndrome, which is characterized by multiple hamartomas and a high proclivity for developing cancer. A series of publications over the past year now suggest a mechanism by which PTEN loss of function results in tumors. PTEN appears to negatively control the phosphoinositide 3-kinase signaling pathway for regulation of cell growth and survival by dephosphorylating the 3 position of phosphoinositides.
Figures

References
-
- Weinberg R A. Cell. 1995;81:323–330. - PubMed
-
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1947. - PubMed
-
- Steck P A, Pershouse M A, Jasser S A, Yung W K, Lin H, Ligon A H, Langford L A, Baumgard M L, Hattier T, Davis T, et al. Nat Genet. 1997;15:356–362. - PubMed
-
- Li D-M, Sun H. Cancer Res. 1997;57:2124–2129. - PubMed
-
- Liu W, James C D, Frederick L, Alderete B E, Jenkins R B. Cancer Res. 1997;57:5254–5257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

