A molecular trigger of lipid binding-induced opening of a helix bundle exchangeable apolipoprotein
- PMID: 10200268
- PMCID: PMC16338
- DOI: 10.1073/pnas.96.8.4366
A molecular trigger of lipid binding-induced opening of a helix bundle exchangeable apolipoprotein
Abstract
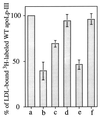
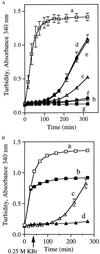
Apolipophorin III (apoLp-III) from the sphinx moth, Manduca sexta, is a helix bundle protein that interacts reversibly with lipoproteins. Its five elongated amphipathic alpha-helices are organized in an antiparallel fashion, with helices 3 and 4 connected by a short 6-residue (PDVEKE) linker helix, termed helix 3'. Upon interaction with lipoproteins, apoLp-III opens to expose a continuous hydrophobic interior. It was postulated that helix bundle opening is preceded by an initiation step wherein helix 3' serves to recognize available lipoprotein surface binding sites. To test this hypothesis, helix 3' was replaced by residues that have a propensity to form a type I beta-turn, NPNG. This mutant apoLp-III was defective in lipoprotein binding assays. To define a more precise mode of interaction, the relevance of the presence of the hydrophobic Val-97 flanked by Asp-96 and Glu-98 was investigated by site-directed mutagenesis. V97N and D96N/V97N/E98Q apoLp-III were unable to compete with wild-type apoLp-III to initiate an interaction with lipoproteins, whereas D96N/E98Q apoLp-III was as competent as wild-type apoLp-III. The results suggest that Val-97 is critical, whereas Asp-96 and Glu-98 are irrelevant for initiating binding to lipoproteins. A model of binding is presented wherein apoLp-III is oriented with the helix 3' end of the molecule juxtaposed to the lipoprotein surface. Recognition of lipoprotein surface hydrophobic defects by Val-97 triggers opening of the helix bundle and facilitates formation of a stable binding interaction.
Figures



Similar articles
-
Fluorescence studies of exchangeable apolipoprotein-lipid interactions. Superficial association of apolipophorin III with lipoprotein surfaces.J Biol Chem. 1998 Jan 16;273(3):1403-8. doi: 10.1074/jbc.273.3.1403. J Biol Chem. 1998. PMID: 9430675
-
Disulfide bond engineering to monitor conformational opening of apolipophorin III during lipid binding.J Biol Chem. 1996 Oct 25;271(43):26855-62. doi: 10.1074/jbc.271.43.26855. J Biol Chem. 1996. PMID: 8900168
-
Interaction of an exchangeable apolipoprotein with phospholipid vesicles and lipoprotein particles. Role of leucines 32, 34, and 95 in Locusta migratoria apolipophorin III.J Biol Chem. 1999 Jul 30;274(31):21804-10. doi: 10.1074/jbc.274.31.21804. J Biol Chem. 1999. PMID: 10419496
-
Merck Frosst award lecture 1995. La conference Merck Frosst 1995. Structural studies of lipoproteins and their apolipoprotein components.Biochem Cell Biol. 1996;74(2):155-64. doi: 10.1139/o96-016. Biochem Cell Biol. 1996. PMID: 9213424 Review.
-
Apolipophorin III: role model apolipoprotein.Insect Biochem Mol Biol. 2006 Apr;36(4):231-40. doi: 10.1016/j.ibmb.2006.01.001. Epub 2006 Jan 18. Insect Biochem Mol Biol. 2006. PMID: 16551537 Review.
Cited by
-
Apolipophorin-III mediates antiplasmodial epithelial responses in Anopheles gambiae (G3) mosquitoes.PLoS One. 2010 Nov 2;5(11):e15410. doi: 10.1371/journal.pone.0015410. PLoS One. 2010. PMID: 21072214 Free PMC article.
-
The N-terminus of apolipoprotein A-V adopts a helix bundle molecular architecture.Biochemistry. 2008 Aug 19;47(33):8768-74. doi: 10.1021/bi800515c. Epub 2008 Jul 25. Biochemistry. 2008. PMID: 18652480 Free PMC article.
-
Alternative lipid mobilization: the insect shuttle system.Mol Cell Biochem. 2002 Oct;239(1-2):113-9. Mol Cell Biochem. 2002. PMID: 12479576 Review.
-
The helix bundle: a reversible lipid binding motif.Comp Biochem Physiol A Mol Integr Physiol. 2010 Feb;155(2):123-33. doi: 10.1016/j.cbpa.2009.09.009. Epub 2009 Sep 19. Comp Biochem Physiol A Mol Integr Physiol. 2010. PMID: 19770066 Free PMC article. Review.
-
Structural basis for the conformational adaptability of apolipophorin III, a helix-bundle exchangeable apolipoprotein.Proc Natl Acad Sci U S A. 2002 Feb 5;99(3):1188-93. doi: 10.1073/pnas.032565999. Epub 2002 Jan 29. Proc Natl Acad Sci U S A. 2002. PMID: 11818551 Free PMC article.
References
-
- Segrest J P, Garber D W, Brouillette C G, Harvey S C, Anantharamaiah G M. Adv Protein Chem. 1994;45:303–369. - PubMed
-
- Fielding C J, Fielding P E. J Lipid Res. 1995;36:211–228. - PubMed
-
- Van der Horst D J. Biochim Biophys Acta. 1990;1047:195–211. - PubMed
-
- Ryan R O. Curr Opin Struct Biol. 1994;4:499–506.
-
- Soulages J L, Wells M A. Adv Protein Chem. 1994;45:371–415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

