Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by preexisting poxvirus immunity
- PMID: 10200293
- PMCID: PMC16363
- DOI: 10.1073/pnas.96.8.4512
Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by preexisting poxvirus immunity
Abstract
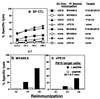
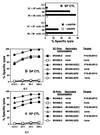
Overcoming preexisting immunity to vaccinia virus in the adult population is a key requirement for development of otherwise potent recombinant vaccinia vaccines. Based on our observation that s.c. immunization with vaccinia induces cellular and antibody immunity to vaccinia only in systemic lymphoid tissue and not in mucosal sites, we hypothesized that the mucosal immune system remains naive to vaccinia and therefore amenable to immunization with recombinant vaccinia vectors despite earlier vaccinia exposure. We show that mucosal immunization of vaccinia-immune BALB/c mice with recombinant vaccinia expressing HIV gp160 induced specific serum antibody and strong HIV-specific cytotoxic T lymphocyte responses. These responses occurred not only in mucosal but also in systemic lymphoid tissue, whereas systemic immunization was ineffective under these circumstances. In this context, intrarectal immunization was more effective than intranasal immunization. Boosting with a second dose of recombinant vaccinia was also more effective via the mucosal route. The systemic HIV-specific cytotoxic T lymphocyte response was enhanced by coadministration of IL-12 at the mucosal site. These results also demonstrate the independent compartmentalization of the mucosal versus systemic immune systems and the asymmetric trafficking of lymphocytes between them. This approach to circumvent previous vaccinia immunity may be useful for induction of protective immunity against infectious diseases and cancer in the sizable populations with preexisting immunity to vaccinia from smallpox vaccination.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

