Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging
- PMID: 10200306
- PMCID: PMC16376
- DOI: 10.1073/pnas.96.8.4586
Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging
Abstract
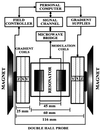
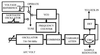
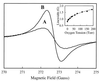
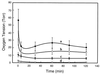
EPR imaging has emerged as an important tool for noninvasive three-dimensional (3D) spatial mapping of free radicals in biological tissues. Spectral-spatial EPR imaging enables mapping of the spectral information at each spatial position, and, from the observed line width, the localized tissue oxygenation can be mapped. We report the development of EPR imaging instrumentation enabling 3D spatial and spectral-spatial EPR imaging of small animals. This instrumentation, along with the use of a biocompatible charcoal oximetry-probe suspension, enabled 3D spatial imaging of the gastrointestinal (GI) tract, along with mapping of oxygenation in living mice. By using these techniques, the oxygen tension was mapped at different levels of the GI tract from the stomach to the rectum. The results clearly show the presence of a marked oxygen gradient from the proximal to the distal GI tract, which decreases after respiratory arrest. This technique for in vivo mapping of oxygenation is a promising method, enabling the noninvasive imaging of oxygen within the normal GI tract. This method should be useful in determining the alterations in oxygenation associated with disease.
Figures




References
-
- Sheridan W G, Lowndes R H, Young H L. Am J Surg. 1990;159:314–319. - PubMed
-
- Knudson M M, Bermudez K M, Doyle C A, Mackersie R C, Hopf H P, Morabito D. J Trauma. 1997;42:608–616. - PubMed
-
- Hauser C J, Locke R R, Kao H W, Patterson J, Zipser R D. J Lab Clin Med. 1988;112:68–71. - PubMed
-
- Cooper G J, Sherry K M, Thorpe J A. Eur J Cardiothorac Surg. 1995;9:158–160. - PubMed
-
- Larsen P N, Moesgaard F, Naver L, Rosenberg J, Gottrup F, Kirkegaard P, Helledie N. Scand J Gastroenterol. 1991;26:409–418. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

