An intronic enhancer containing an N-box motif is required for synapse- and tissue-specific expression of the acetylcholinesterase gene in skeletal muscle fibers
- PMID: 10200313
- PMCID: PMC16383
- DOI: 10.1073/pnas.96.8.4627
An intronic enhancer containing an N-box motif is required for synapse- and tissue-specific expression of the acetylcholinesterase gene in skeletal muscle fibers
Abstract
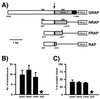
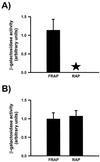
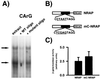
mRNAs encoding acetylcholinesterase (AChE; EC 3.1.1.7) are highly concentrated within the postsynaptic sarcoplasm of adult skeletal muscle fibers, where their expression is markedly influenced by nerve-evoked electrical activity and trophic factors. To determine whether transcriptional regulatory mechanisms account for the synaptic accumulation of AChE transcripts at the mammalian neuromuscular synapse, we cloned a 5.3-kb DNA fragment that contained the 5' regulatory region of the rat AChE gene and generated several constructs in which AChE promoter fragments were placed upstream of the reporter gene lacZ and a nuclear localization signal (nls). Using a recently described transient expression assay system in intact skeletal muscle, we show that this AChE promoter fragment directs the synapse-specific expression of the reporter gene. Deletion analysis revealed that a 499-bp fragment located in the first intron of the AChE gene is essential for expression in muscle fibers. Further analysis showed that sequences contained within this intronic fragment were (i) functionally independent of position and orientation and (ii) inactive in hematopoietic cells. Disruption of an N-box motif located within this DNA fragment reduced by more than 80% the expression of the reporter gene in muscle fibers. In contrast, mutation of an adjacent CArG element had no effect on nlsLacZ expression. Taken together, these results indicate that a muscle-specific enhancer is present within the first intron of the AChE gene and that an intronic N-box is essential for the regulation of AChE along skeletal muscle fibers.
Figures


Similar articles
-
Role of intronic E- and N-box motifs in the transcriptional induction of the acetylcholinesterase gene during myogenic differentiation.J Biol Chem. 2001 May 18;276(20):17603-9. doi: 10.1074/jbc.M100916200. Epub 2001 Feb 22. J Biol Chem. 2001. PMID: 11279154
-
Neural regulation of acetylcholinesterase mRNAs at mammalian neuromuscular synapses.J Cell Biol. 1994 Nov;127(4):1061-9. doi: 10.1083/jcb.127.4.1061. J Cell Biol. 1994. PMID: 7962068 Free PMC article.
-
Transcriptional regulation of acetylcholinesterase-associated collagen ColQ: differential expression in fast and slow twitch muscle fibers is driven by distinct promoters.J Biol Chem. 2004 Jun 25;279(26):27098-107. doi: 10.1074/jbc.M402596200. Epub 2004 Apr 21. J Biol Chem. 2004. PMID: 15102835
-
Control levels of acetylcholinesterase expression in the mammalian skeletal muscle.Chem Biol Interact. 1999 May 14;119-120:309-19. doi: 10.1016/s0009-2797(99)00041-1. Chem Biol Interact. 1999. PMID: 10421466 Review.
-
Nerve-derived trophic factors and DNA elements controlling expression of genes encoding synaptic proteins in skeletal muscle fibers.Can J Appl Physiol. 1998 Aug;23(4):366-76. doi: 10.1139/h98-021. Can J Appl Physiol. 1998. PMID: 9677433 Review.
Cited by
-
A comparative investigation on H3K27ac enhancer activities in the brain and liver tissues between wild boars and domesticated pigs.Evol Appl. 2022 Aug 16;15(8):1281-1290. doi: 10.1111/eva.13461. eCollection 2022 Aug. Evol Appl. 2022. PMID: 36051459 Free PMC article.
-
The role of muscle activation pattern and calcineurin in acetylcholinesterase regulation in rat skeletal muscles.J Neurosci. 2007 Jan 31;27(5):1106-13. doi: 10.1523/JNEUROSCI.4182-06.2007. J Neurosci. 2007. PMID: 17267565 Free PMC article.
-
Mapping and analysis of a spatiotemporal H3K27ac and gene expression spectrum in pigs.Sci China Life Sci. 2022 Aug;65(8):1517-1534. doi: 10.1007/s11427-021-2034-5. Epub 2022 Jan 27. Sci China Life Sci. 2022. PMID: 35122624
-
Properties of non-coding DNA and identification of putative cis-regulatory elements in Theileria parva.BMC Genomics. 2008 Dec 3;9:582. doi: 10.1186/1471-2164-9-582. BMC Genomics. 2008. PMID: 19055776 Free PMC article.
-
The Long Intron 1 of Growth Hormone Gene from Reeves' Turtle (Chinemys reevesii) Correlates with Negatively Regulated GH Expression in Four Cell Lines.Int J Mol Sci. 2016 Apr 12;17(4):543. doi: 10.3390/ijms17040543. Int J Mol Sci. 2016. PMID: 27077853 Free PMC article.
References
-
- Massoulié J, Pezzementi L, Bon S, Krejci E, Vallette F-M. Prog Neurobiol. 1993;41:31–91. - PubMed
-
- Taylor P, Radic Z. Annu Rev Pharmacol Toxicol. 1994;34:281–320. - PubMed
-
- Boudreau-Larivière C, Gisiger V, Michel R N, Hubatsch D A, Jasmin B J. Am J Physiol. 1997;272:C68–C76. - PubMed
-
- Jasmin B J, Lee R K, Rotundo R L. Neuron. 1993;11:467–477. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

