An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons
- PMID: 10200319
- PMCID: PMC16389
- DOI: 10.1073/pnas.96.8.4662
An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons
Abstract
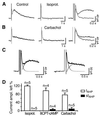
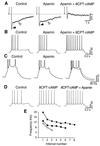
In hippocampal and other cortical neurons, action potentials are followed by afterhyperpolarizations (AHPs) generated by the activation of small-conductance Ca2+-activated K+ channels (SK channels). By shaping the neuronal firing pattern, these AHPs contribute to the regulation of excitability and to the encoding function of neurons. Here we report that CA1 pyramidal neurons express an AHP current that is suppressed by apamin and is involved in the control of repetitive firing. This current presents distinct kinetic and pharmacological features, and it is modulated differently than the apamin-insensitive slow AHP current. Furthermore, our in situ hybridizations show that the apamin-sensitive SK subunits are expressed in CA1 pyramidal neurons, providing a potential molecular correlate to the apamin-sensitive AHP current. Altogether, these results clarify the discrepancy between the reported high density of apamin-binding sites in the CA1 region and the apparent lack of an apamin-sensitive current in CA1 pyramidal neurons, and they may explain the effects of this toxin on hippocampal synaptic plasticity and learning.
Figures



Similar articles
-
Stability and plasticity of intrinsic membrane properties in hippocampal CA1 pyramidal neurons: effects of internal anions.J Physiol. 2007 Feb 1;578(Pt 3):799-818. doi: 10.1113/jphysiol.2006.124586. Epub 2006 Nov 30. J Physiol. 2007. PMID: 17138601 Free PMC article.
-
β3-Adrenergic receptor-dependent modulation of the medium afterhyperpolarization in rat hippocampal CA1 pyramidal neurons.J Neurophysiol. 2019 Mar 1;121(3):773-784. doi: 10.1152/jn.00334.2018. Epub 2019 Jan 9. J Neurophysiol. 2019. PMID: 30625002 Free PMC article.
-
Small conductance Ca2+-activated K+ channels as targets of CNS drug development.Curr Drug Targets CNS Neurol Disord. 2004 Jun;3(3):161-7. doi: 10.2174/1568007043337472. Curr Drug Targets CNS Neurol Disord. 2004. PMID: 15180477 Review.
-
Medium afterhyperpolarization and firing pattern modulation in interneurons of stratum radiatum in the CA3 hippocampal region.J Neurophysiol. 2001 May;85(5):1986-97. doi: 10.1152/jn.2001.85.5.1986. J Neurophysiol. 2001. PMID: 11353015
-
SK (KCa2) channels do not control somatic excitability in CA1 pyramidal neurons but can be activated by dendritic excitatory synapses and regulate their impact.J Neurophysiol. 2008 Nov;100(5):2589-604. doi: 10.1152/jn.90433.2008. Epub 2008 Aug 6. J Neurophysiol. 2008. PMID: 18684909
Cited by
-
Apical SK potassium channels and Ca2+-dependent anion secretion in endometrial epithelial cells.J Physiol. 2008 Feb 1;586(3):717-26. doi: 10.1113/jphysiol.2007.142877. Epub 2007 Nov 29. J Physiol. 2008. PMID: 18048454 Free PMC article.
-
Ca2+ -activated K+ channels of the BK-type in the mouse brain.Histochem Cell Biol. 2006 Jun;125(6):725-41. doi: 10.1007/s00418-005-0124-7. Epub 2005 Dec 14. Histochem Cell Biol. 2006. PMID: 16362320
-
Developmental sensitivity of hippocampal interneurons to ethanol: involvement of the hyperpolarization-activated current, Ih.J Neurophysiol. 2009 Jan;101(1):67-83. doi: 10.1152/jn.90557.2008. Epub 2008 Oct 29. J Neurophysiol. 2009. PMID: 18971298 Free PMC article.
-
The riluzole derivative 2-amino-6-trifluoromethylthio-benzothiazole (SKA-19), a mixed KCa2 activator and NaV blocker, is a potent novel anticonvulsant.Neurotherapeutics. 2015 Jan;12(1):234-49. doi: 10.1007/s13311-014-0305-y. Neurotherapeutics. 2015. PMID: 25256961 Free PMC article.
-
Molecular and cellular basis of small--and intermediate-conductance, calcium-activated potassium channel function in the brain.Cell Mol Life Sci. 2008 Oct;65(20):3196-217. doi: 10.1007/s00018-008-8216-x. Cell Mol Life Sci. 2008. PMID: 18597044 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

