Molecular insights into the physiology of the 'thin film' of airway surface liquid
- PMID: 10200413
- PMCID: PMC2269304
- DOI: 10.1111/j.1469-7793.1999.0631u.x
Molecular insights into the physiology of the 'thin film' of airway surface liquid
Abstract
The epithelia that line the airways of the lung exhibit two general functions: (1) airway epithelia in all regions 'defend' the lung against infectious and noxious agents; and (2) airway epithelia in the proximal regions replenish water lost from airway surfaces, i.e. the 'insensible water loss', consequent to conditioning inspired air. How airway epithelia perform both functions, and co-ordinate them in health and disease, is the subject of this review.
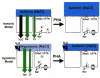
Figures



References
-
- Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–205. - PubMed
-
- Bear CE, Li C, Kartner N, Bridges RJ, Jensen TJ, Ramjeesingh M, Riordan JR. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR) Cell. 1992;68:809–818. - PubMed
-
- Boucher RC. Human airway ion transport (Part 1) American Journal of Respiratory and Critical Care Medicine. 1994;150:271–281. - PubMed
-
- Boucher RC, Stutts MJ, Bromberg PA, Gatzy JT. Regional differences in airway surface liquid composition. Journal of Applied Physiology. 1981;50:613–620. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

