Single channel properties of human alpha3 AChRs: impact of beta2, beta4 and alpha5 subunits
- PMID: 10200416
- PMCID: PMC2269285
- DOI: 10.1111/j.1469-7793.1999.0657u.x
Single channel properties of human alpha3 AChRs: impact of beta2, beta4 and alpha5 subunits
Abstract
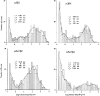
1. We performed single channel analysis on human alpha3 acetylcholine receptors (AChRs) in Xenopus oocytes and native AChRs from the human neuroblastoma cell line IMR-32. alpha3 AChRs exhibit channel properties that reflect subunit composition. 2. alpha3beta2 AChR open times were 0.71 +/- 0.14 and 3.5 +/- 0.4 ms with a predominant conductance of 26 pS. alpha3beta4 AChRs had open times of 1.4 +/- 0.2 and 6.5 +/- 0.8 ms and a predominant conductance of 31 pS. Burst times were 0.82 +/- 0.12 and 5.3 +/- 0.7 ms for alpha3beta2 and 1.7 +/- 0.1 and 16 +/- 1 ms for alpha3beta4. Desensitization was faster for AChRs with the beta2 subunit than for those with the beta4 subunit. 3. One open time for alpha3alpha5beta2 AChRs (5.5 +/- 0.3 ms) was different from those of alpha3beta2 AChRs. For alpha3alpha5beta4 AChRs, an additional conductance, open time and burst time (36 pS, 22 +/- 3 ms and 43 +/- 4 ms, respectively) were different from those for alpha3beta4 AChRs. 4. alpha3 AChRs were inhibited by hexamethonium or mecamylamine. The rate constants for block of alpha3beta4 by hexamethonium and of alpha3beta2 by mecamylamine were 1.2 x 107 and 4.6 x 107 M-1 s-1, respectively. 5. AChRs from IMR-32 cells had a predominant conductance of 32 pS and open times of 1.5 +/- 0.3 and 9.6 +/- 1.2 ms. These properties were most similar to those of alpha3beta4 AChRs expressed in oocytes. Antibodies revealed that 5 +/- 2 % of IMR-32 alpha3 AChRs contained alpha5 subunits and 6 +/- 2 % contained beta2 subunits. IMR-32 alpha3 AChRs are primarily alpha3beta4 AChRs.
Figures









Similar articles
-
Functional properties of human nicotinic AChRs expressed by IMR-32 neuroblastoma cells resemble those of alpha3beta4 AChRs expressed in permanently transfected HEK cells.J Gen Physiol. 2001 Nov;118(5):563-82. doi: 10.1085/jgp.118.5.563. J Gen Physiol. 2001. PMID: 11696612 Free PMC article.
-
Chronic nicotine treatment up-regulates human alpha3 beta2 but not alpha3 beta4 acetylcholine receptors stably transfected in human embryonic kidney cells.J Biol Chem. 1998 Oct 30;273(44):28721-32. doi: 10.1074/jbc.273.44.28721. J Biol Chem. 1998. PMID: 9786868
-
Assembly of human neuronal nicotinic receptor alpha5 subunits with alpha3, beta2, and beta4 subunits.J Biol Chem. 1996 Jul 26;271(30):17656-65. doi: 10.1074/jbc.271.30.17656. J Biol Chem. 1996. PMID: 8663494
-
Recombinant nicotinic receptors, expressed in Xenopus oocytes, do not resemble native rat sympathetic ganglion receptors in single-channel behaviour.J Physiol. 1997 Apr 1;500 ( Pt 1)(Pt 1):123-38. doi: 10.1113/jphysiol.1997.sp022004. J Physiol. 1997. PMID: 9097938 Free PMC article.
-
Role of non-neuronal nicotinic acetylcholine receptors in angiogenesis.Int J Biochem Cell Biol. 2009 Jul;41(7):1441-51. doi: 10.1016/j.biocel.2009.01.013. Epub 2009 Jan 29. Int J Biochem Cell Biol. 2009. PMID: 19401144 Review.
Cited by
-
Decreased α4β2 nicotinic receptor number in the absence of mRNA changes suggests post-transcriptional regulation in the spontaneously hypertensive rat model of ADHD.J Neurochem. 2011 Oct;119(1):240-50. doi: 10.1111/j.1471-4159.2011.07415.x. Epub 2011 Sep 1. J Neurochem. 2011. PMID: 21824140 Free PMC article.
-
Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats.J Neurosci. 2003 Jun 1;23(11):4712-6. doi: 10.1523/JNEUROSCI.23-11-04712.2003. J Neurosci. 2003. PMID: 12805310 Free PMC article.
-
Functional characterization of the α5(Asn398) variant associated with risk for nicotine dependence in the α3β4α5 nicotinic receptor.Mol Pharmacol. 2011 Nov;80(5):818-27. doi: 10.1124/mol.111.073841. Epub 2011 Aug 19. Mol Pharmacol. 2011. PMID: 21856741 Free PMC article.
-
Functional properties of human nicotinic AChRs expressed by IMR-32 neuroblastoma cells resemble those of alpha3beta4 AChRs expressed in permanently transfected HEK cells.J Gen Physiol. 2001 Nov;118(5):563-82. doi: 10.1085/jgp.118.5.563. J Gen Physiol. 2001. PMID: 11696612 Free PMC article.
-
Nicotinic effects on excitatory field potentials recorded from the immature CA3 area of rat hippocampal slices.Exp Brain Res. 2003 Oct;152(3):353-60. doi: 10.1007/s00221-003-1546-x. Epub 2003 Jul 26. Exp Brain Res. 2003. PMID: 12898092
References
-
- Alkondon M, Reinhardt S, Lobron C, Hermsen B, Maelicke A, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. II. The rundown and inward rectification of agonist-elicited whole-cell currents and identification of receptor subunits by in situ hybridization. Journal of Pharmacology and Experimental Therapeutics. 1994;271:494–506. - PubMed
-
- Cachelin AB, Jaggi R. β subunits determine the time course of desensitization in rat α3 neuronal nicotinic acetylcholine receptors. Pflügers Archiv. 1991;419:579–582. - PubMed
-
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors hα2β2, hα2β4, hα3β2, hα3β4, hα4β2, hα4β4 and hα7 expressed in Xenopus oocytes. Journal of Pharmacology and Experimental Therapeutics. 1997;280:346–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

