Somatic and prejunctional nicotinic receptors in cultured rat sympathetic neurones show different agonist profiles
- PMID: 10200422
- PMCID: PMC2269288
- DOI: 10.1111/j.1469-7793.1999.0739u.x
Somatic and prejunctional nicotinic receptors in cultured rat sympathetic neurones show different agonist profiles
Abstract
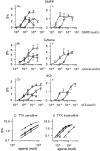
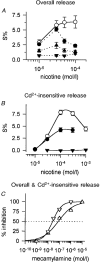
1. The release of [3H]-noradrenaline ([3H]-NA) in response to nicotinic acetylcholine receptor (nAChR) agonists was compared with agonist-induced currents in cultured rat superior cervical ganglion (SCG) neurones. 2. [3H]-NA release in response to high concentrations of nicotinic agonists was reduced, but not fully inhibited, by the presence of either tetrodotoxin (TTX) or Cd2+ to block voltage-gated Na+ or Ca2+ channels, respectively. We used the component of transmitter release that remained in the presence of these substances (named TTX- or Cd2+-insensitive release) to pharmacologically characterize nAChRs in proximity to the sites of vesicular exocytosis (prejunctional receptors). Prejunctional nAChRs were activated by nicotinic agonists with a rank order of potency of dimethylphenylpiperazinium iodide (DMPP) > nicotine > cytisine > ACh, and with EC50 values ranging from 22 microM (DMPP) to 110 microM (ACh). 3. [3H]-NA release in response to low concentrations of nAChR agonists was fully inhibited by the presence of either TTX or Cd2+ (named TTX- or Cd2+-sensitive release). TTX-sensitive release was triggered by nicotinic agonists with a rank order of potency of DMPP > cytisine approximately nicotine approximately ACh, which due to its similarity to TTX-insensitive release indicates that it might also be triggered by prejunctional-type nAChRs. The EC50 values for TTX (Cd2+)-sensitive release were less than 10 microM for all four agonists. 4. By contrast to transmitter release, somatic nAChRs as seen by patch clamp recordings were most potently activated by cytisine, with a rank order of potency of cytisine > nicotine approximately DMPP > ACh. EC50 values for the induction of currents exceeded 20 microM for all four agonists. 5. The nicotinic antagonist mecamylamine potently inhibited all transmitter release in response to nicotine. alpha-Bungarotoxin (alpha-BuTX) was, on the other hand, without significant effect on nicotine-induced TTX-insensitive release. The competitive antagonist dihydro-beta-erythroidine (DHbetaE) caused rightward shifts of the dose-response curves for both TTX-sensitive and TTX-insensitive transmitter release as well as for currents in response to nicotine, with pA2 values ranging from 4.03 to 4.58. 6. Due to clear differences in the pharmacology of agonists we propose that nAChRs of distinct subunit composition are differentially targeted to somatic or axonal domains.
Figures





References
-
- Boehm S, Huck S. α2-Adrenoreceptor-mediated inhibition of acetylcholine-induced noradrenaline release from rat sympathetic neurons: an action at voltage-gated Ca2+ channels. Neuroscience. 1995;69:221–231. - PubMed
-
- Boehm S, Huck S. Receptors controlling transmitter release from sympathetic neurons in vitro. Progress in Neurobiology. 1997;51:225–242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

