Beta-adrenergic agonists regulate cell membrane fluctuations of human erythrocytes
- PMID: 10200425
- PMCID: PMC2269310
- DOI: 10.1111/j.1469-7793.1999.0781u.x
Beta-adrenergic agonists regulate cell membrane fluctuations of human erythrocytes
Abstract
1. Mechanical fluctuations of the cell membrane (CMFs) in human erythrocytes reflect the bending deformability of the membrane-skeleton complex. These fluctuations were monitored by time-dependent light scattering from a small area ( approximately 0. 25 microm2) of the cell surface by a method based on point dark field microscopy. 2. Exposure of red blood cells (RBCs) to adrenaline (epinephrine) and isoproterenol (isoprenaline) resulted in up to a 45 % increase in the maximal fluctuation amplitude and up to a 35 % increase in the half-width of the amplitude distribution. The power spectra of membrane fluctuations of control and treated cells revealed that adrenaline stimulated only the low frequency component (0.3-3 Hz). Analysis of the dose-response curves of beta-adrenergic agonists yielded an EC50 of 5 x 10-9 and 1 x 10-11 M for adrenaline and isoproterenol, respectively. Propranolol had an inhibitory effect on the stimulatory effect of isoproterenol. These findings show a potency order of propranolol > isoproterenol > adrenaline. 3. The stimulatory effect of adrenaline was a temporal one, reaching its maximal level after 20-30 min but being abolished after 60 min. However, in the presence of 3-isobutyl-1-methylxanthine, a partial stimulatory effect was maintained even after 60 min. Pentoxifylline and 8-bromo-cAMP elevated CMFs. However, exposure of ATP-depleted erythrocytes to adrenaline or 8-bromo-cAMP did not yield any elevation in CMFs. These findings suggest that the beta-agonist effect on CMFs is transduced via a cAMP-dependent pathway. 4. Deoxygenation decreased CMFs and filterability of erythrocytes by approximately 30 %. The stimulatory effect of isoproterenol on CMFs was 2.2-fold higher in deoxygenated RBCs than in oxygenated cells. 5. Exposure of RBCs to adrenaline resulted in a concentration-dependent increase in RBC filterability, demonstrating a linear relationship between CMFs and filterability, under the same exposure conditions to adrenaline. These findings suggest that beta-adrenergic agonists may improve passage of erythrocytes through microvasculature, enhancing oxygen delivery to tissues, especially under situations of reduced oxygen tension for periods longer than 20 min.
Figures
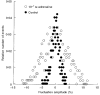

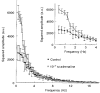

 ) of 10−9 M isoproterenol for 20 min, at 37 °C. The amplitudes are given by δImax/I (%; means ±
) of 10−9 M isoproterenol for 20 min, at 37 °C. The amplitudes are given by δImax/I (%; means ±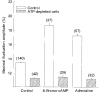
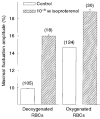
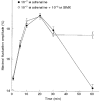
 ) RBCs were incubated for 20 min with 10−4 M 8-bromo-cAMP and 10−7 M adrenaline, at 37 °C. The amplitudes are given by δImax/I (%; means ±
) RBCs were incubated for 20 min with 10−4 M 8-bromo-cAMP and 10−7 M adrenaline, at 37 °C. The amplitudes are given by δImax/I (%; means ±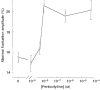

Similar articles
-
Effect of oxygen tension on catecholamine-induced formation of cAMP and on swelling of carp red blood cells.Am J Physiol. 1990 Nov;259(5 Pt 1):C723-6. doi: 10.1152/ajpcell.1990.259.5.C723. Am J Physiol. 1990. PMID: 1978570
-
Relative efficacy and potency of beta-adrenoceptor agonists for generating cAMP in human lymphocytes.Chest. 1996 Jan;109(1):194-200. doi: 10.1378/chest.109.1.194. Chest. 1996. PMID: 8549185
-
Adrenergic responses of R. ridibunda red cells.J Exp Zool. 1996 Oct 15;276(3):175-85. doi: 10.1002/(SICI)1097-010X(19961015)276:3<175::AID-JEZ1>3.0.CO;2-K. J Exp Zool. 1996. PMID: 8914277
-
Catecholamine regulation of human erythrocyte membrane protein kinase.J Clin Invest. 1979 Aug;64(2):534-40. doi: 10.1172/JCI109491. J Clin Invest. 1979. PMID: 222812 Free PMC article.
-
Interaction of epinephrine with isolated rabbit tracheal epithelial cells.Am J Physiol. 1986 Aug;251(2 Pt 1):C209-15. doi: 10.1152/ajpcell.1986.251.2.C209. Am J Physiol. 1986. PMID: 2874740
Cited by
-
Squeezing for Life - Properties of Red Blood Cell Deformability.Front Physiol. 2018 Jun 1;9:656. doi: 10.3389/fphys.2018.00656. eCollection 2018. Front Physiol. 2018. PMID: 29910743 Free PMC article. Review.
-
Erythrocytes membrane fluidity changes induced by adenylyl cyclase cascade activation: study using fluorescence recovery after photobleaching.Eur Biophys J. 2024 May;53(4):239-247. doi: 10.1007/s00249-024-01707-x. Epub 2024 Apr 16. Eur Biophys J. 2024. PMID: 38625405 Free PMC article.
-
Red blood cell β-adrenergic receptors contribute to diet-induced energy expenditure by increasing O2 supply.JCI Insight. 2017 Jul 20;2(14):e93367. doi: 10.1172/jci.insight.93367. eCollection 2017 Jul 20. JCI Insight. 2017. PMID: 28724789 Free PMC article.
-
An on-chip deformability checker demonstrates that the severity of iron deficiency is associated with increased deformability of red blood cells.Sci Rep. 2025 Jun 6;15(1):19994. doi: 10.1038/s41598-025-05483-2. Sci Rep. 2025. PMID: 40481296 Free PMC article.
-
Differential dielectroscopic data on the relation of erythrocyte membrane skeleton to erythrocyte deformability and flicker.Eur Biophys J. 2021 Jan;50(1):69-86. doi: 10.1007/s00249-020-01491-4. Epub 2021 Jan 13. Eur Biophys J. 2021. PMID: 33442752
References
-
- Allen JE, Rasmussen H. Human red blood cells: prostaglandin E2, epinephrine and isoproterenol alter deformability. Science. 1971;174:512–514. - PubMed
-
- Backman L. Shape control in human red cell. Journal of Cell Science. 1986;80:281–298. - PubMed
-
- Bagge U, Branemark PI, Karlsson R, Skalak R. Three-dimensional observations of red blood cell deformation in capillaries. Blood Cells. 1980;6:213–239. - PubMed
-
- Bayer R, Plewa S, Borcescu E, Claus W. Filterability of human erythrocytes - drug induced prevention of aging in vitro. Arzneimittel Forschung. 1988;38:1765–1767. - PubMed
-
- Beavo JA, Rogers NL, Crofford OB, Hardman LG, Sutherland EW, Newman EV. Effects of xanthine derivatives on lipolysis and on adenosine 3′,5′-monophosphate phosphodiesterase activity. Molecular Pharmacology. 1970;6:597–603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

