Ca2+-independent phosphorylation of myosin in rat caudal artery and chicken gizzard myofilaments
- PMID: 10200427
- PMCID: PMC2269290
- DOI: 10.1111/j.1469-7793.1999.0805u.x
Ca2+-independent phosphorylation of myosin in rat caudal artery and chicken gizzard myofilaments
Abstract
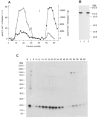
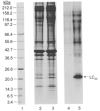
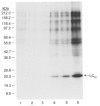
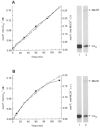
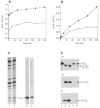
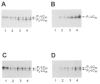
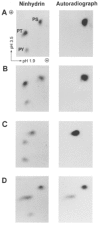
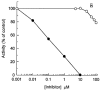
1. Smooth muscle contraction is activated primarily by the Ca2+-calmodulin (CaM)-dependent phosphorylation of the 20 kDa light chains (LC20) of myosin. Activation can also occur in some instances without a change in intracellular free [Ca2+] or indeed in a Ca2+-independent manner. These signalling pathways often involve inhibition of myosin light chain phosphatase and unmasking of basal kinase activity leading to LC20 phosphorylation and contraction. 2. We have used demembranated rat caudal arterial smooth muscle strips and isolated chicken gizzard myofilaments in conjunction with the phosphatase inhibitor microcystin-LR to investigate the mechanism of Ca2+-independent phosphorylation of LC20 and contraction. 3. Treatment of Triton X-100-demembranated rat caudal arterial smooth muscle strips with microcystin at pCa 9 triggered a concentration-dependent contraction that was slower than that induced by pCa 4.5 or 6 but reached comparable steady-state levels of tension. 4. This Ca2+-independent, microcystin-induced contraction correlated with phosphorylation of LC20 at serine-19 and threonine-18. 5. Whereas Ca2+-dependent LC20 phosphorylation and contraction were inhibited by a synthetic peptide (AV25) based on the autoinhibitory domain of myosin light chain kinase (MLCK), Ca2+-independent, microcystin-induced LC20 phosphorylation and contraction were resistant to AV25. 6. Ca2+-independent LC20 kinase activity was also detected in chicken gizzard smooth muscle myofilaments and catalysed phosphorylation of endogenous myosin LC20 at serine-19 and/or threonine-18. This is in contrast to MLCK which phosphorylates threonine-18 only after prior phosphorylation of serine-19. 7. Gizzard Ca2+-independent LC20 kinase could be separated from MLCK by differential extraction from myofilaments and by CaM affinity chromatography. Its activity was resistant to AV25. 8. We conclude that inhibition of smooth muscle myosin light chain phosphatase (MLCP) unmasks the activity of a Ca2+-independent LC20 kinase associated with the myofilaments and distinct from MLCK. This kinase, therefore, probably plays a role in Ca2+ sensitization and Ca2+-independent contraction of smooth muscle in response to stimuli that act via Ca2+-independent pathways, leading to inhibition of MLCP.
Figures
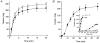


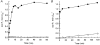
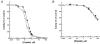

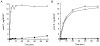



References
-
- Adachi K, Carruthers CA, Walsh MP. Identification of the native form of chicken gizzard myosin light chain kinase with the aid of monoclonal antibodies. Biochemical and Biophysical Research Communications. 1983;115:855–863. - PubMed
-
- Allen BG, Andrea JE, Walsh MP. Identification and characterization of protein kinase Cζ-immunoreactive proteins. Journal of Biological Chemistry. 1994;269:29288–29298. - PubMed
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) Journal of Biological Chemistry. 1996;271:20246–20249. 10.1074/jbc.271.34.20246. - DOI - PubMed
-
- Bresnick AR, Wolff-Long VL, Baumann O, Pollard TD. Phosphorylation on threonine-18 of the regulatory light chain dissociates the ATPase and motor properties of smooth muscle myosin II. Biochemistry. 1995;34:12576–12583. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

