Clinical and financial benefits of rapid bacterial identification and antimicrobial susceptibility testing
- PMID: 10203497
- PMCID: PMC84789
- DOI: 10.1128/JCM.37.5.1415-1418.1999
Clinical and financial benefits of rapid bacterial identification and antimicrobial susceptibility testing
Abstract
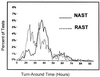
To assess the expected clinical and financial benefits of rapid reporting of microbiology results, we compared patients whose cultured samples were processed in the normal manner to patients whose samples were processed more rapidly due to a minor change in work flow. For the samples tested in the rapid-reporting time period, the vast majority of bacterial identification and antimicrobial susceptibility testing (AST) results were verified with the Vitek system on the same day that they were available. This time period was called rapid AST (RAST). For RAST, a technologist on the evening shift verified the data that became available during that shift. For the control time period, cultures were processed in the normal manner (normal AST [NAST]), which did not include evening-shift verification. For NAST, the results for approximately half of the cultures were verified on the first day that the result was available. The average turnaround time for the reporting of AST results was 39.2 h for RAST and 44.4 h for NAST (5.2 h faster for RAST [P = 0.001]). Subsequently, physicians were able to initiate appropriate antimicrobial therapy sooner for patients whose samples were tested as part of RAST (P = 0.006). The mortality rates were 7. 9 and 9.6% for patients whose samples were tested as part of RAST and NAST, respectively (P = 0.45). The average length of stay was 10. 7 days per patient for RAST and 12.6 days for NAST, a difference of 2.0 days less for RAST (P = 0.006). The average variable cost was $4, 927 per patient for RAST and $6,677 for NAST, a difference of $1,750 less per patient for RAST (P = 0.001). This results in over $4 million in savings in variable costs per year in our hospital.
Figures
References
-
- Barenfanger J, Drake C, Kacich G. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Benefits of rapid reporting in microbiology, C-461; p. 208.
-
- Campo L, Mylotte J M. Use of microbiology reports by physicians in prescribing antimicrobial agents. Am J Med Sci. 1988;296:392–398. - PubMed
-
- Check W. Managed care deeply affecting clinical microbiology. ASM News. 1998;64:495–500.
-
- Dawson-Saunders B, Trapp R. Biostatistics. 2nd ed. Norwalk, Conn: Appleton and Lange; 1994.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources