Second generation of the automated Cobas Amplicor HCV assay improves sensitivity of hepatitis C virus RNA detection and yields results that are more clinically relevant
- PMID: 10203523
- PMCID: PMC84830
- DOI: 10.1128/JCM.37.5.1567-1569.1999
Second generation of the automated Cobas Amplicor HCV assay improves sensitivity of hepatitis C virus RNA detection and yields results that are more clinically relevant
Abstract
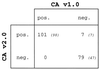
The first and second generations of the Cobas Amplicor HCV assay were compared among patients at risk of hepatitis C virus (HCV) infection. The second-generation test was found to be of greater sensitivity and of good specificity among clinical specimens containing HCV RNA of different genotypes. Finally, this new test is shown to predict the outcome of interferon therapy better.
Figures
References
-
- Albadalejo J, Alonso R, Antinozzi R, Bogard M, Bourgault A-M, Colucci G, Fenner T, Petersen H, Sala E, Vincelette J, Young C. Multicenter evaluation of the COBAS AMPLICOR HCV assay, an integrated PCR system for rapid detection of hepatitis C virus RNA in the diagnostic laboratory. J Clin Microbiol. 1998;36:862–865. - PMC - PubMed
-
- Davis G L, Lau J Y. Factors predictive of a beneficial response to therapy of hepatitis C. Hepatology. 1997;26:122S–127S. - PubMed
-
- DiDomenico N, Link H, Knobel R, Caratsch T, Weschler W, Loewy Z G, Rosenstraus M. COBAS AMPLICOR: fully automated RNA and DNA amplification and detection system for routine diagnostic PCR. Clin Chem. 1996;42:1915–1923. - PubMed
-
- Doglio A, Laffont C, Thyss S, Lefebvre J. Rapid genotyping of hepatitis C virus by direct cycle sequencing of PCR-amplified cDNAs and capillary electrophoresis. Res Virol. 1998;149:219–227. - PubMed
-
- Gerken G, Pontisso P, Roggendorf M, Grazia R M, Simmonds P, Trepo C, Zeuzem S, Colucci G. Clinical evaluation of a single reaction, diagnostic polymerase chain reaction assay for the detection of hepatitis C virus RNA. J Hepatol. 1996;24:33–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

