Conformational restraints revealing bioactive beta-bend structures for halpha CGRP8-37 at the CGRP2 receptor of the rat prostatic vas deferens
- PMID: 10205004
- PMCID: PMC1565896
- DOI: 10.1038/sj.bjp.0702432
Conformational restraints revealing bioactive beta-bend structures for halpha CGRP8-37 at the CGRP2 receptor of the rat prostatic vas deferens
Abstract
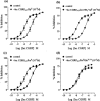
1. The main aim of this study was to identify putative beta-bends and the role of the N- and C-terminus in the CGRP receptor antagonist halpha CGRP8-37, which was measured against halpha CGRP inhibition of twitch responses in the rat prostatic vas deferens. 2. With a bend-biasing residue (proline) at position 16 in halpha CGRP8-37 (10(-5) M) an inactive compound was produced, while alanine at the same position retained antagonist activity (apparent pKB 5.6+/-0.1 at 10(-5) M). Proline at position 19 within halpha CGRP8-37 (10(-5) M) was an antagonist (apparent pKB 5.8+/-0.1). 3. Incorporation of a bend-forcing structure (beta-turn dipeptide or BTD) at either positions 19,20 or 33,34 in halpha CGRP8-37 (10(-5) M) antagonized halpha CGRP responses (apparent pKB 6.0+/-0.1 and 6.1+/-0.1, respectively). Replacement by BTD at both positions 19,20 and 33,34 within halpha CGRP8-37 competitively antagonized responses to halpha CGRP (pA2 6.2; Schild plot slope 1.0+/-0.1). 4. Halpha CGRP8-37 analogues (10(-5) M), substituted at the N-terminus by either glycine8, or des-NH2 valine8 or proline8 were all antagonists against halpha CGRP (apparent pKB 6.1+/-0.1, 6.5+/-0.1 and 6.1+/-0.1, respectively), while halpha CGRP8-37 (10(-5) M) substituted in three places by proline8 and glutamic acid10,14 was inactive. 5. Replacement of the C-terminus by alanine amide37 in halpha CGRP8-37 (10(-5) M) failed to antagonize halpha CGRP responses. 6. Peptidase inhibitors did not alter either the agonist potency of halpha CGRP or the antagonist affinities of halpha CGRP8-37 BTD19,20 and 33,34 and halpha CGRP8-37 Gly8 (against halpha CGRP responses). 7. In conclusion, two beta-bends at positions 18-21 and 32-35 are compatible with high affinity by BTD and is the first approach of modelling the bioactive structure of halpha CGRP8-37. Further, the N-terminus of halpha CGRP8-37 is not essential for antagonism, while the C-terminus interacts directly with CGRP receptor binding sites of the rat vas deferens.
Figures
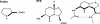
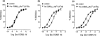
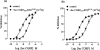
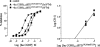
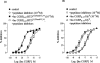
Similar articles
-
Bioactive beta-bend structures for the antagonist halpha CGRP(8 - 37) at the CGRP(1) receptor of the rat pulmonary artery.Br J Pharmacol. 2000 Mar;129(5):1049-55. doi: 10.1038/sj.bjp.0703152. Br J Pharmacol. 2000. PMID: 10696108 Free PMC article.
-
CGRP(2) receptor in the internal anal sphincter of the rat: implications for CGRP receptor classification.Br J Pharmacol. 2000 May;130(2):464-70. doi: 10.1038/sj.bjp.0703315. Br J Pharmacol. 2000. PMID: 10807687 Free PMC article.
-
Pharmacological characterization of CGRP receptors mediating relaxation of the rat pulmonary artery and inhibition of twitch responses of the rat vas deferens.Br J Pharmacol. 1998 Apr;123(8):1673-83. doi: 10.1038/sj.bjp.0701783. Br J Pharmacol. 1998. PMID: 9605575 Free PMC article.
-
Multiple receptors for calcitonin gene-related peptide and amylin on guinea-pig ileum and vas deferens.Br J Pharmacol. 1996 Mar;117(6):1362-8. doi: 10.1111/j.1476-5381.1996.tb16737.x. Br J Pharmacol. 1996. PMID: 8882637 Free PMC article.
-
Pharmacology of receptors for calcitonin gene-related peptide and amylin.Trends Pharmacol Sci. 1995 Dec;16(12):424-8. doi: 10.1016/s0165-6147(00)89093-8. Trends Pharmacol Sci. 1995. PMID: 8578616 Review.
Cited by
-
Three-dimensional structure and orientation of rat islet amyloid polypeptide protein in a membrane environment by solution NMR spectroscopy.J Am Chem Soc. 2009 Jun 17;131(23):8252-61. doi: 10.1021/ja9010095. J Am Chem Soc. 2009. PMID: 19456151 Free PMC article.
-
CGRP physiology, pharmacology, and therapeutic targets: migraine and beyond.Physiol Rev. 2023 Apr 1;103(2):1565-1644. doi: 10.1152/physrev.00059.2021. Epub 2022 Dec 1. Physiol Rev. 2023. PMID: 36454715 Free PMC article. Review.
-
Bioactive beta-bend structures for the antagonist halpha CGRP(8 - 37) at the CGRP(1) receptor of the rat pulmonary artery.Br J Pharmacol. 2000 Mar;129(5):1049-55. doi: 10.1038/sj.bjp.0703152. Br J Pharmacol. 2000. PMID: 10696108 Free PMC article.
-
CGRP(2) receptor in the internal anal sphincter of the rat: implications for CGRP receptor classification.Br J Pharmacol. 2000 May;130(2):464-70. doi: 10.1038/sj.bjp.0703315. Br J Pharmacol. 2000. PMID: 10807687 Free PMC article.
-
New insights into protein-protein interaction modulators in drug discovery and therapeutic advance.Signal Transduct Target Ther. 2024 Dec 6;9(1):341. doi: 10.1038/s41392-024-02036-3. Signal Transduct Target Ther. 2024. PMID: 39638817 Free PMC article. Review.
References
-
- BOULANGER Y., KHIAT A., CHEN Y., SENECAL L., TU Y., ST-PIERRE S., FOURNIER A. Structure of human calcitonin gene-related peptide and of its antagonist human calcitonin gene-related peptide 8-37 by nmr and molecular modelling. Peptide Res. 1995;8:206–213. - PubMed
-
- BOULANGER Y., KHIAT A., LAROCQUE A., FOURNIER A., ST-PIERRE S. Structural comparison of alanine-substituted analogues of the calcitonin gene-related peptide 8-37. Int. J. Peptide Protein Res. 1996;47:477–483. - PubMed
-
- BRAIN D., WILLIAMS T.J., TIPPINS J.R., MORRIS H.R., MACYNTYRE I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. - PubMed
-
- BREEZE A.L., HARVEY T.S., BAZZO R., CAMPBELL I.D. Solution structure of human calcitonin gene-related peptide by 1H-NMR and distance geometry with restrained molecular dynamics. Biochemistry. 1991;30:575–582. - PubMed
-
- CAPRINO L.A., HAN G.Y. The 9-Fluorenylmethoxycarbonyl amino-protecting group. J. Org. Chem. 1972;37:3404–3409.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

