Randomised trial of mycophenolate mofetil versus azathioprine for treatment of chronic active Crohn's disease
- PMID: 10205197
- PMCID: PMC1727513
- DOI: 10.1136/gut.44.5.625
Randomised trial of mycophenolate mofetil versus azathioprine for treatment of chronic active Crohn's disease
Abstract
Background: Crohn's disease is a chronic inflammatory disease of the alimentary tract. Azathioprine is an effective agent in the management of chronic active Crohn's disease leading to long term remission of disease activity. Such treatment leads to limited efficacy or side effects in a small subset of patients.
Aims: To compare efficacy and side effects of treatment with azathioprine plus corticosteroids versus mycophenolate mofetil (MMF) plus corticosteroids in patients with chronic active Crohn's disease.
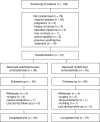
Methods: Seventy patients with chronic active Crohn's disease (Crohn's disease activity index (CDAI) greater than 150) were randomised for treatment with azathioprine/cortisone or MMF/cortisone. Corticosteroid dosage was tapered according to a standard protocol. Disease activity was monitored by clinical scores after one, two, three, and six months.
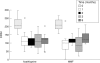
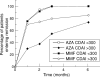
Results: Treatment of patients with moderately active (CDAI 150-300) Crohn's disease with MMF/cortisone led to a significant reduction in clinical activity scores comparable to treatment with azathioprine/cortisone. Treatment of patients with highly active Crohn's disease (CDAI greater than 300) with MMF/cortisone caused significant suppression of clinical activity earlier than azathioprine/cortisone treatment. Treatment with MMF/cortisone was associated with few adverse effects.
Conclusion: Treatment of chronic active Crohn's disease with MMF plus cortisone appears to be effective and well tolerated and should be considered in patients allergic to azathioprine or in whom azathioprine has failed.
Figures
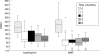

Comment in
-
Is mycophenolate mofetil a new alternative in the treatment of inflammatory bowel disease?Gut. 1999 May;44(5):592-3. doi: 10.1136/gut.44.5.592. Gut. 1999. PMID: 10205187 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
