Cost comparison of predictive genetic testing versus conventional clinical screening for familial adenomatous polyposis
- PMID: 10205208
- PMCID: PMC1727488
- DOI: 10.1136/gut.44.5.698
Cost comparison of predictive genetic testing versus conventional clinical screening for familial adenomatous polyposis
Abstract
Background: Mutations of the APC gene cause familial adenomatous polyposis (FAP), a hereditary colorectal cancer predisposition syndrome.
Aims: To conduct a cost comparison analysis of predictive genetic testing versus conventional clinical screening for individuals at risk of inheriting FAP, using the perspective of a third party payer.
Methods: All direct health care costs for both screening strategies were measured according to time and motion, and the expected costs evaluated using a decision analysis model.
Results: The baseline analysis predicted that screening a prototype FAP family would cost $4975/ pound3109 by molecular testing and $8031/ pound5019 by clinical screening strategy, when family members were monitored with the same frequency of clinical surveillance (every two to three years). Sensitivity analyses revealed that the genetic testing approach is cost saving for key variables including the kindred size, the age of screening onset, and the cost of mutation identification in a proband. However, if the APC mutation carriers were monitored at an increased (annual) frequency, the cost of the genetic screening strategy increased to $7483/ pound4677 and was especially sensitive to variability in age of onset of screening, family size, and cost of genetic testing of at risk relatives.
Conclusions: In FAP kindreds, a predictive genetic testing strategy costs less than conventional clinical screening, provided that the frequency of surveillance is identical using either strategy. An additional significant benefit is the elimination of unnecessary colonic examinations for those family members found to be non-carriers.
Figures
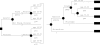
Similar articles
-
Cost analysis of alternative approaches to colorectal screening in familial adenomatous polyposis.Gastroenterology. 1998 May;114(5):893-901. doi: 10.1016/s0016-5085(98)70308-7. Gastroenterology. 1998. PMID: 9558276
-
Economic evaluation of the familial cancer programme in Western Australia: predictive genetic testing for familial adenomatous polyposis and hereditary non-polyposis colorectal carcinoma.Community Genet. 2006;9(2):98-106. doi: 10.1159/000091487. Community Genet. 2006. PMID: 16612060
-
Presymptomatic direct detection of adenomatous polyposis coli (APC) gene mutations in familial adenomatous polyposis.Hum Genet. 1993 May;91(4):307-11. doi: 10.1007/BF00217347. Hum Genet. 1993. PMID: 8388848
-
Familial adenomatous polyposis.Best Pract Res Clin Gastroenterol. 2009;23(2):197-207. doi: 10.1016/j.bpg.2009.02.010. Best Pract Res Clin Gastroenterol. 2009. PMID: 19414146 Review.
-
Detecting mutations in the APC gene in familial adenomatous polyposis (FAP).Curr Protoc Hum Genet. 2006 Aug;Chapter 10:Unit 10.8. doi: 10.1002/0471142905.hg1008s50. Curr Protoc Hum Genet. 2006. PMID: 18428386 Review.
Cited by
-
Cost-utility analysis of genetic screening in families of patients with germline MUTYH mutations.BMC Med Genet. 2007 Jul 2;8:42. doi: 10.1186/1471-2350-8-42. BMC Med Genet. 2007. PMID: 17605803 Free PMC article.
-
Incorporating Cascade Effects of Genetic Testing in Economic Evaluation: A Scoping Review of Methodological Challenges.Children (Basel). 2021 Apr 27;8(5):346. doi: 10.3390/children8050346. Children (Basel). 2021. PMID: 33925765 Free PMC article.
-
Hereditary colorectal cancer registries in Canada: report from the Colorectal Cancer Association of Canada consensus meeting; Montreal, Quebec; October 28, 2011.Curr Oncol. 2013 Oct;20(5):273-8. doi: 10.3747/co.20.1566. Curr Oncol. 2013. PMID: 24155632 Free PMC article.
-
Predictive genetic tests and health system costs.CMAJ. 2003 Apr 15;168(8):989-91. CMAJ. 2003. PMID: 12695382 Free PMC article. No abstract available.
-
Retinoblastoma: genetic testing versus conventional clinical screening in India.Mol Diagn. 2004;8(4):237-43. doi: 10.1007/BF03260068. Mol Diagn. 2004. PMID: 15887979
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
