The natural history of histologically proved drug induced liver disease
- PMID: 10205214
- PMCID: PMC1727509
- DOI: 10.1136/gut.44.5.731
The natural history of histologically proved drug induced liver disease
Abstract
Background: The long term outcome of drug related liver disease is unknown.
Aims: To study the natural history of histologically proved drug induced hepatotoxicity.
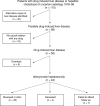
Methods: 110 patients with liver biopsies coded either as drug induced liver disease or hepatitis/cholestasis of unknown aetiology were identified from hospital records 1978-1996. Review of case notes and histology identified 44 patients with definite drug induced hepatotoxicity. Forty surviving patients were invited to attend a follow up clinic. History, examination, full liver screen, and isotope and ultrasound liver scans were repeated in all patients. Repeat liver biopsies were offered to patients with abnormal liver tests.
Results: Presentation at index biopsy was jaundice in 24 patients, abnormal liver tests in 17, and hepatic failure in three. Antibiotics (n=13) and non-steroidal anti-inflammatory drugs (n=11) were the most common drugs implicated. Initial histology showed acute hepatitis in six, chronic hepatitis in 20, and cholestasis in 18. At 1-19 years (median 5 years) follow up, 13/33 (39%) patients had persistent significant abnormalities in their liver blood tests and/or scans. Three of the five repeat liver biopsies performed showed significant abnormalities. Factors predicting persistence or development of chronic liver disease were fibrosis and continued exposure to the drug.
Conclusions: Drugs should be considered in the differential diagnosis of abnormal liver function and/or histology, as failure to withdraw the offending drug is associated with a high risk of persistent liver damage.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical