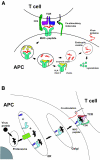
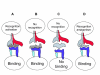
Mechanisms of immune escape in viral hepatitis
- PMID: 10205220
- PMCID: PMC1727502
- DOI: 10.1136/gut.44.5.759
Mechanisms of immune escape in viral hepatitis
Figures
Similar articles
-
[Non-A, non-B viral hepatitis and cellular immunity].Nihon Rinsho. 1981 Oct;39(10):3277-82. Nihon Rinsho. 1981. PMID: 6803027 Review. Japanese. No abstract available.
-
[Immunopathogenic aspects of chronic viral hepatitis].Rev Clin Esp. 1996 Mar;196(3):179-84. Rev Clin Esp. 1996. PMID: 8650389 Review. Spanish. No abstract available.
-
[Trends in research of the development, chronicity, and cellular immunity of viral hepatitis].Nihon Rinsho. 1983;41(4):795-800. Nihon Rinsho. 1983. PMID: 6411951 Review. Japanese. No abstract available.
-
[Immune mechanisms in inflammatory liver diseases].Internist (Berl). 1985 Oct;26(10):607-13. Internist (Berl). 1985. PMID: 3935596 German. No abstract available.
-
Blood T and B cells in patients with acute viral hepatitis A, B and non-A non-B.Boll Ist Sieroter Milan. 1982;61(5):375-82. Boll Ist Sieroter Milan. 1982. PMID: 6821443
Cited by
-
In Silico Prediction of Human Leukocytes Antigen (HLA) Class II Binding Hepatitis B Virus (HBV) Peptides in Botswana.Viruses. 2020 Jul 6;12(7):731. doi: 10.3390/v12070731. Viruses. 2020. PMID: 32640609 Free PMC article.
-
Upregulation of major histocompatibility complex class I on liver cells by hepatitis C virus core protein via p53 and TAP1 impairs natural killer cell cytotoxicity.J Virol. 2003 Aug;77(15):8299-309. doi: 10.1128/jvi.77.15.8299-8309.2003. J Virol. 2003. PMID: 12857899 Free PMC article.
-
Evidence for lack of cross-genotype protection of CD4+ T cell responses during chronic hepatitis C virus infection.Clin Exp Immunol. 2003 Jan;131(1):122-9. doi: 10.1046/j.1365-2249.2003.02033.x. Clin Exp Immunol. 2003. PMID: 12519395 Free PMC article.
-
Molecular signatures associated with HCV-induced hepatocellular carcinoma and liver metastasis.PLoS One. 2013;8(2):e56153. doi: 10.1371/journal.pone.0056153. Epub 2013 Feb 18. PLoS One. 2013. PMID: 23441164 Free PMC article.
-
Integrated Clinical, Molecular and Immunological Characterization of Pulmonary Sarcomatoid Carcinomas Reveals an Immune Escape Mechanism That May Influence Therapeutic Strategies.Int J Mol Sci. 2023 Jun 23;24(13):10558. doi: 10.3390/ijms241310558. Int J Mol Sci. 2023. PMID: 37445733 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
