FKLF, a novel Krüppel-like factor that activates human embryonic and fetal beta-like globin genes
- PMID: 10207080
- PMCID: PMC84149
- DOI: 10.1128/MCB.19.5.3571
FKLF, a novel Krüppel-like factor that activates human embryonic and fetal beta-like globin genes
Abstract
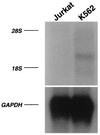
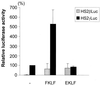
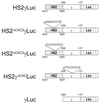
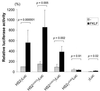
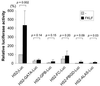
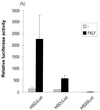
A cDNA encoding a novel Krüppel-type zinc finger protein, FKLF, was cloned from fetal globin-expressing human fetal erythroid cells. The deduced polypeptide sequence composed of 512 amino acids revealed that, like Sp1 and EKLF, FKLF has three contiguous zinc fingers at the near carboxyl-terminal end. A long amino-terminal domain is characterized by the presence of two acidic and two proline-rich regions. Reverse transcription (RT)-PCR assays using various cell lines demonstrated that the FKLF mRNA is expressed predominantly in erythroid cells. FKLF message is detectable by RT-PCR in fetal liver but not in adult bone marrow cells. As predicted from its structural features, FKLF is a transcriptional activator. In luciferase assays FKLF activated the gamma- and epsilon-globin gene promoters, and, to a much lower degree, the beta-globin promoter. Studies of HS2-gamma gene reporter constructs carrying CACCC box deletions revealed that the CACCC box sequence of the gamma gene promoter mediates the activation of the gamma gene by FKLF. Other erythroid promoters (GATA-1, glycophorin B, ferrochelatase, porphobilinogen deaminase, and 5-aminolevulinate synthase) containing CACCC elements or GC-rich potential Sp1-binding sites were activated minimally, if at all, by FKLF, indicating that FKLF is not a general activator of genes carrying the CACCC motifs. Transfection of K562 cells with FKLF cDNA enhanced the expression of the endogenous epsilon- and gamma-globin genes, suggesting an in vivo role of FKLF in fetal and embryonic globin gene expression. Our results indicate that the protein potentially encoded by the FKLF cDNA acts as a transcriptional activator of embryonic and fetal beta-like globin genes.
Figures





References
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994.
-
- Bieker J J. Role of erythroid Krüppel-like factor (EKLF) in erythroid-specific transcription. In: Stamatoyannopoulos G, editor. Molecular biology of hemoglobin switching. Andover, United Kingdom: Intercept; 1995. pp. 231–241.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
