HERF1, a novel hematopoiesis-specific RING finger protein, is required for terminal differentiation of erythroid cells
- PMID: 10207104
- PMCID: PMC84222
- DOI: 10.1128/MCB.19.5.3808
HERF1, a novel hematopoiesis-specific RING finger protein, is required for terminal differentiation of erythroid cells
Abstract
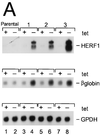
The AML1/core binding factor beta (CBFbeta) transcription factor is essential for definitive hematopoiesis; however, the downstream pathways through which it functions remain incompletely defined. Using a differential cloning approach to define components of this pathway, we have identified a novel gene designated HERF1 (for hematopoietic RING finger 1), whose expression during development is dependent on the presence of functional AML1/CBFbeta. HERF1 contains a tripartite RING finger-B box-alpha-helical coiled-coil domain and a C-terminal region homologous to the ret proto-oncogene-encoded finger protein. Expression of HERF1 during embryogenesis coincides with the appearance of definitive erythropoiesis and in adult mice is restricted to erythroid cells, increasing 30-fold during terminal differentiation. Importantly, inhibition of HERF1 expression blocked terminal erythroid differentiation of the murine erythroleukemia cell line MEL, whereas its overexpression induced erythroid maturation. These results suggest an important role for this protein in erythropoiesis.
Figures

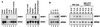
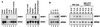
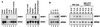




References
-
- Borden K L B, Freemont P S. The RING finger domain: a recent example of a sequence-structure family. Curr Opin Struct Biol. 1996;6:395–401. - PubMed
-
- Borden K L B, Martin S R, O’Reilly N J, Lally J M, Reddy B A, Etkin L D, Freemont P S. Characterisation of a novel cysteine/histidine-rich metal binding domain from Xenopus nuclear factor XNF7. FEBS Lett. 1993;335:255–260. - PubMed
-
- Chang K S, Fan Y H, Andreeff M, Liu J, Mu Z M. The PML gene encodes a phosphoprotein associated with the nuclear matrix. Blood. 1995;85:3646–3653. - PubMed
-
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
