Expression of Trk receptors in the developing mouse trigeminal ganglion: in vivo evidence for NT-3 activation of TrkA and TrkB in addition to TrkC
- PMID: 10207144
- PMCID: PMC2710120
- DOI: 10.1242/dev.126.10.2191
Expression of Trk receptors in the developing mouse trigeminal ganglion: in vivo evidence for NT-3 activation of TrkA and TrkB in addition to TrkC
Abstract
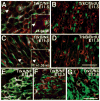
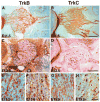
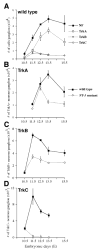
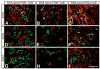
Animals lacking neurotrophin-3 (NT-3) are born with deficits in almost all sensory ganglia. Among these, the trigeminal ganglion is missing 70% of the normal number of neurons, a deficit which develops during the major period of neurogenesis between embryonic stages (E) 10.5 and E13.5. In order to identify the mechanisms for this deficit, we used antisera specific for TrkA, TrkB, and TrkC to characterize and compare the expression patterns of each Trk receptor in trigeminal ganglia of wild type and NT-3 mutants between E10.5 and E15.5. Strikingly, TrkA, TrkB, and TrkC proteins appear to be exclusively associated with neurons, not precursors. While some neurons show limited co-expression of Trk receptors at E11.5, by E13. 5 each neuron expresses only one Trk receptor. Neuronal birth dating and cell counts show that in wild-type animals all TrkB- and TrkC-expressing neurons are generated before E11.5, while the majority of TrkA-expressing neurons are generated between E11.5 and E13.5. In mice lacking NT-3, the initial formation of the ganglion, as assessed at E10.5, is similar to that in wild-type animals. At E11.5, however, the number of TrkC-expressing neurons is dramatically reduced and the number of TrkC-immunopositive apoptotic profiles is markedly elevated. By E13.5, TrkC-expressing neurons are virtually eliminated. At E11.5, compared to wild type, the number of TrkB-expressing neurons is also reduced and the number of TrkB immunoreactive apoptotic profiles is increased. TrkA neurons are also reduced in the NT-3 mutants, but the major deficit develops between E12.5 and E13.5 when elevated numbers of TrkA-immunoreactive apoptotic profiles are detected. Normal numbers of TrkA- and TrkB-expressing neurons are seen in a TrkC-deficient mutant. Therefore, our data provide evidence that NT-3 supports the survival of TrkA-, TrkB- and TrkC-expressing neurons in the trigeminal ganglion by activating directly each of these receptors in vivo.
Figures


Similar articles
-
Timing of neuronal death in trkA, trkB and trkC mutant embryos reveals developmental changes in sensory neuron dependence on Trk signalling.Development. 1996 Oct;122(10):3255-61. doi: 10.1242/dev.122.10.3255. Development. 1996. PMID: 8898237
-
POU domain factor Brn-3a controls the differentiation and survival of trigeminal neurons by regulating Trk receptor expression.Development. 1999 Jul;126(13):2869-82. doi: 10.1242/dev.126.13.2869. Development. 1999. PMID: 10357931 Free PMC article.
-
Neurotrophin receptor genes are expressed in distinct patterns in developing dorsal root ganglia.J Neurosci. 1993 Sep;13(9):4029-41. doi: 10.1523/JNEUROSCI.13-09-04029.1993. J Neurosci. 1993. PMID: 8366358 Free PMC article.
-
Structural and functional properties of the TRK family of neurotrophin receptors.Ann N Y Acad Sci. 1995 Sep 7;766:442-58. doi: 10.1111/j.1749-6632.1995.tb26693.x. Ann N Y Acad Sci. 1995. PMID: 7486690 Review.
-
The Trk family of neurotrophin receptors.J Neurobiol. 1994 Nov;25(11):1386-403. doi: 10.1002/neu.480251107. J Neurobiol. 1994. PMID: 7852993 Review.
Cited by
-
AAV1.NT-3 gene therapy attenuates spontaneous autoimmune peripheral polyneuropathy.Gene Ther. 2016 Jan;23(1):95-102. doi: 10.1038/gt.2015.67. Epub 2015 Jun 30. Gene Ther. 2016. PMID: 26125608 Free PMC article.
-
Targeted deletion of numb and numblike in sensory neurons reveals their essential functions in axon arborization.Genes Dev. 2005 Jan 1;19(1):138-51. doi: 10.1101/gad.1246005. Epub 2004 Dec 14. Genes Dev. 2005. PMID: 15598981 Free PMC article.
-
Local neurotrophin effects on central trigeminal axon growth patterns.Brain Res Dev Brain Res. 2004 Jul 19;151(1-2):55-66. doi: 10.1016/j.devbrainres.2004.03.017. Brain Res Dev Brain Res. 2004. PMID: 15246692 Free PMC article.
-
Loss of Elp1 disrupts trigeminal ganglion neurodevelopment in a model of familial dysautonomia.Elife. 2022 Jun 17;11:e71455. doi: 10.7554/eLife.71455. Elife. 2022. PMID: 35713404 Free PMC article.
-
The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB.J Neurosci. 2000 Sep 15;20(18):6888-97. doi: 10.1523/JNEUROSCI.20-18-06888.2000. J Neurosci. 2000. PMID: 10995833 Free PMC article.
References
-
- Barbacid M. The trk family of neurotrophin receptors. J Neurobiol. 1994;11:1386–1403. - PubMed
-
- Barker PA, Lomen-Hoerth C, Gensch EM, Meakin SO, Glass DJ, Shooter EM. Tissue-specific alternative splicing generates two isoforms of the TrkA receptor. J Biol Chem. 1993;268:15150–15157. - PubMed
-
- Buchman VL, Davies AM. Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons. Development. 1993;118:989–1001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

