Molecular and functional analysis of SDCT2, a novel rat sodium-dependent dicarboxylate transporter
- PMID: 10207168
- PMCID: PMC408276
- DOI: 10.1172/JCI5392
Molecular and functional analysis of SDCT2, a novel rat sodium-dependent dicarboxylate transporter
Abstract
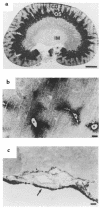
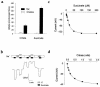
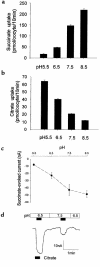
Kidney proximal tubule cells take up Krebs cycle intermediates for metabolic purposes and for secretion of organic anions through dicarboxylate/organic anion exchange. Alteration in reabsorption of citrate is closely related to renal stone formation. The presence of distinct types of sodium-coupled dicarboxylate transporters has been postulated on either side of the polarized epithelial membrane in the kidney proximal tubule. Using a PCR-based approach, we isolated a novel member of the sodium-dependent dicarboxylate/sulfate transporter called SDCT2. SDCT2 is a 600-amino acid residue protein that has 47-48% amino acid identity to SDCT1 and NaDC-1, previously identified in kidney and intestine. Northern analysis gave a single band of 3.3 kb for SDCT2 in kidney, liver, and brain. In situ hybridization revealed that SDCT2 is prominently expressed in kidney proximal tubule S3 segments and in perivenous hepatocytes, consistent with the sites of high-affinity dicarboxylate transport identified based on vesicle studies. A signal was also detected in the meningeal layers of the brain. SDCT2 expressed in Xenopus oocytes mediated sodium-dependent transport of di- and tricarboxylates with substrate preference for succinate rather than citrate, but excluding monocarboxylates. SDCT2, unlike SDCT1, displayed a unique pH dependence for succinate transport (optimal pH 7.5-8.5) and showed a high affinity for dimethylsuccinate, two features characteristic of basolateral transport. These data help to interpret the mechanisms of renal citrate transport, their alteration in pathophysiological conditions, and their role in the elimination of organic anions and therapeutic drugs.
Figures


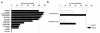

References
-
- Murer, H., Manganel, M., and Roch-Ramel, F. 1992. Tubular transport of monocarboxylates, Krebs cycle intermediates, and inorganic sulfate. In Handbook of physiology: renal physiology. E.E. Windhager, editor. American Physiological Society. Bethesda, MD. 2165–2188.
-
- Hamm LL. Renal handling of citrate. Kidney Int. 1990;38:728–735. - PubMed
-
- Brennan S, Hering-Smith K, Hamm LL. Effect of pH on citrate reabsorption in the proximal convoluted tubule. Am J Physiol. 1988;255:F301–F306. - PubMed
-
- Wright EM. Transport of carboxylic acids by renal membrane vesicles. Annu Rev Physiol. 1985;47:127–141. - PubMed
-
- Wright SH, Kippen I, Wright EM. Effect of pH on the transport of Krebs cycle intermediates in renal brush border membranes. Biochim Biophys Acta. 1982;684:287–290. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

