RAE1 is a shuttling mRNA export factor that binds to a GLEBS-like NUP98 motif at the nuclear pore complex through multiple domains
- PMID: 10209021
- PMCID: PMC2133102
- DOI: 10.1083/jcb.145.2.237
RAE1 is a shuttling mRNA export factor that binds to a GLEBS-like NUP98 motif at the nuclear pore complex through multiple domains
Abstract
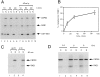
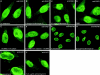
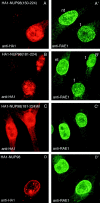
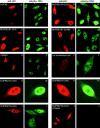
Gle2p is implicated in nuclear export of poly(A)+ RNA and nuclear pore complex (NPC) structure and distribution in Saccharomyces cerevisiae. Gle2p is anchored at the nuclear envelope (NE) via a short Gle2p-binding motif within Nup116p called GLEBS. The molecular mechanism by which Gle2p and the Gle2p-Nup116p interaction function in mRNA export is unknown. Here we show that RAE1, the mammalian homologue of Gle2p, binds to a GLEBS-like NUP98 motif at the NPC through multiple domains that include WD-repeats and a COOH-terminal non-WD-repeat extension. This interaction is direct, as evidenced by in vitro binding studies and chemical cross-linking. Microinjection experiments performed in Xenopus laevis oocytes demonstrate that RAE1 shuttles between the nucleus and the cytoplasm and is exported from the nucleus in a temperature-dependent and RanGTP-independent manner. Docking of RAE1 to the NE is highly dependent on new mRNA synthesis. Overexpression of the GLEBS-like motif also inhibits NE binding of RAE1 and induces nuclear accumulation of poly(A)+ RNA. Both effects are abrogated either by the introduction of point mutations in the GLEBS-like motif or by overexpression of RAE1, indicating a direct role for RAE1 and the NUP98-RAE1 interaction in mRNA export. Together, our data suggest that RAE1 is a shuttling transport factor that directly contributes to nuclear export of mRNAs through its ability to anchor to a specific NUP98 motif at the NPC.
Figures





References
-
- Amberg DC, Goldstein AL, Cole CN. Isolation and characterization of RAT1: an essential gene of Saccharomyces cerevisiaerequired for the efficient nucleocytoplasmic trafficking of mRNA. Genes Dev. 1992;6:1173–1189. - PubMed
-
- Arts GJ, Fornerod M, Mattaj IW. Identification of a nuclear export receptor for tRNA. Curr Biol. 1998;8:305–314. - PubMed
-
- Audigier Y. Assays for studying functional properties of in vitro translated Gs alpha subunit. Methods Enzymol. 1994;237:239–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

