Prefoldin-nascent chain complexes in the folding of cytoskeletal proteins
- PMID: 10209023
- PMCID: PMC2133115
- DOI: 10.1083/jcb.145.2.265
Prefoldin-nascent chain complexes in the folding of cytoskeletal proteins
Abstract
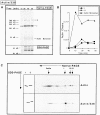
In vitro transcription/translation of actin cDNA and analysis of the translation products by native-PAGE was used to study the maturation pathway of actin. During the course of actin synthesis, several distinct actin-containing species were observed and the composition of each determined by immunological procedures. After synthesis of the first approximately 145 amino acids, the nascent ribosome-associated actin chain binds to the recently identified heteromeric chaperone protein, prefoldin (PFD). PFD remains bound to the relatively unfolded actin polypeptide until its posttranslational delivery to cytosolic chaperonin (CCT). We show that alpha- and beta-tubulin follow a similar maturation pathway, but to date find no evidence for an interaction between PFD and several noncytoskeletal proteins. We conclude that PFD functions by selectively targeting nascent actin and tubulin chains pending their transfer to CCT for final folding and/or assembly.
Figures






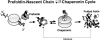
References
-
- Anfinsen CB. Principles that govern the folding of protein chains. Science. 1973;181:223–230. - PubMed
-
- Beckmann RP, Mizzen LA, Welch WJ. Interactions of hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248:850–854. - PubMed
-
- Blattler DP, Garner F, Van Slyke K, Bradley A. Quantitative electrophoresis in polyacrylamide gels of 2–40% J Chromatogr. 1972;64:147–155.
-
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

