An essential role for katanin in severing microtubules in the neuron
- PMID: 10209026
- PMCID: PMC2133110
- DOI: 10.1083/jcb.145.2.305
An essential role for katanin in severing microtubules in the neuron
Abstract
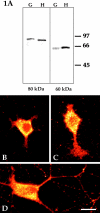
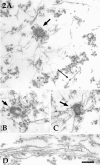
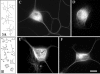
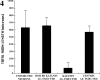
Several lines of evidence suggest that microtubules are nucleated at the neuronal centrosome, and then released for transport into axons and dendrites. Here we sought to determine whether the microtubule-severing protein known as katanin mediates microtubule release from the neuronal centrosome. Immunomicroscopic analyses on cultured sympathetic neurons show that katanin is present at the centrosome, but is also widely distributed throughout the neuron. Microinjection of an antibody that inactivates katanin results in a dramatic accumulation of microtubules at the centrosome, indicating that katanin is indeed required for microtubule release from the centrosome. However, the antibody also causes an inhibition of axon outgrowth that is more immediate than expected on this basis alone. It may be that katanin severs microtubules throughout the cell body to keep them sufficiently short to be efficiently transported into developing processes. Consistent with this idea, there were significantly fewer free ends of microtubules in the cell bodies of neurons that had been injected with the katanin antibody compared with controls. These results indicate that microtubule-severing by katanin is essential for releasing microtubules from the neuronal centrosome, and also for regulating the length of the microtubules after their release.
Figures

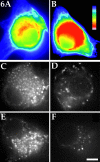
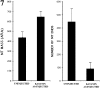
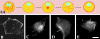
References
-
- Ahmad FJ, Baas PW. Microtubules released from the neuronal centrosome are transported into the axon. J Cell Sci. 1995;108:2761–2769. - PubMed
-
- Ahmad FJ, Joshi HC, Frohlich V, Baas PW. Inhibition of microtubule nucleation at the centrosome compromises axon growth. Neuron. 1994;12:271–280. - PubMed
-
- Baas PW. Microtubules and axonal growth. Curr Opin Cell Biol. 1997;9:29–36. - PubMed
-
- Baas PW. Microtubules and neuronal polarity: lessons from mitosis. Neuron. 1999;22:23–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

