Superantigen-induced T cell responses in acute rheumatic fever and chronic rheumatic heart disease patients
- PMID: 10209512
- PMCID: PMC1905222
- DOI: 10.1046/j.1365-2249.1999.00853.x
Superantigen-induced T cell responses in acute rheumatic fever and chronic rheumatic heart disease patients
Abstract
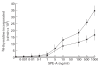
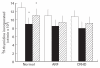
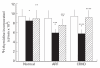
CD4+ and CD8+ T cells from healthy donors, acute rheumatic fever (ARF) and chronic rheumatic heart disease (CRHD) patients responded variably to a superantigen from Streptococcus pyogenes--Streptococcal pyrogenic erythrogenic toxin A (SPE-A). In vitro culture of CD4+ T cells from ARF patients (CD4-ARF) with SPE-A exhibited a Th1 type of response as they produced high levels of IL-2, while CD4+ T cells from CRHD patients (CD4-RHD) secreted IL-4 and IL-10 in large amounts, i.e. Th2 type of cytokine profile. The skewing of human CD4+ T cells (in response to SPE-A stimulation) to Th1 or Th2 type reflects the role of the two subsets in a disorder with differing intensities at the two extremes of the spectrum. Moreover, the anergy induction experiments revealed that CD8-ARF and CD8-RHD undergo anergy (to different extents), whereas CD4+ T cells do not, in response to re-stimulation by SPE-A. These results initially demonstrate that both CD4+ and CD8+ T cells respond differentially to SPE-A, and hence it is an important observation with respect to the pathogenesis of ARF/CRHD. Anergy in CD8+ T cells in the presence of SPE-A in vitro goes a step further to show the clinical relevance of these cells and their possible role in suppression of the disease.
Figures
Similar articles
-
T cell subsets: an integral component in pathogenesis of rheumatic heart disease.Immunol Res. 2018 Feb;66(1):18-30. doi: 10.1007/s12026-017-8978-z. Immunol Res. 2018. PMID: 29170852 Review.
-
Enhancement of IL-1, IL-2 production and IL-2 receptor generation in patients with acute rheumatic fever and active rheumatic heart disease; a prospective study.Clin Exp Immunol. 1993 Mar;91(3):429-36. doi: 10.1111/j.1365-2249.1993.tb05920.x. Clin Exp Immunol. 1993. PMID: 8095193 Free PMC article.
-
Antibody levels and in vitro lymphoproliferative responses to Streptococcus pyogenes erythrogenic toxin A and mitogen of patients with rheumatic fever.J Clin Microbiol. 1991 Sep;29(9):1789-94. doi: 10.1128/jcm.29.9.1789-1794.1991. J Clin Microbiol. 1991. PMID: 1774298 Free PMC article.
-
Immune responsiveness during disease progression from acute rheumatic fever to chronic rheumatic heart disease.Microbes Infect. 2012 Oct;14(12):1111-7. doi: 10.1016/j.micinf.2012.07.003. Epub 2012 Jul 14. Microbes Infect. 2012. PMID: 22796386
-
Rheumatic fever: how S. pyogenes-primed peripheral T cells trigger heart valve lesions.Ann N Y Acad Sci. 2005 Jun;1051:132-40. doi: 10.1196/annals.1361.054. Ann N Y Acad Sci. 2005. PMID: 16126952 Review.
Cited by
-
Overexpression of transforming growth factor-beta 1 in the valvular fibrosis of chronic rheumatic heart disease.J Korean Med Sci. 2008 Feb;23(1):41-8. doi: 10.3346/jkms.2008.23.1.41. J Korean Med Sci. 2008. PMID: 18303197 Free PMC article.
-
Detecting sub-clinical disease activity in patients with chronic rheumatic valvular heart disease.Indian Heart J. 2021 May-Jun;73(3):313-318. doi: 10.1016/j.ihj.2021.02.009. Epub 2021 Feb 27. Indian Heart J. 2021. PMID: 34154748 Free PMC article.
-
T cell subsets: an integral component in pathogenesis of rheumatic heart disease.Immunol Res. 2018 Feb;66(1):18-30. doi: 10.1007/s12026-017-8978-z. Immunol Res. 2018. PMID: 29170852 Review.
-
The Role of Inflammation and Oxidative Stress in Rheumatic Heart Disease.Int J Mol Sci. 2022 Dec 13;23(24):15812. doi: 10.3390/ijms232415812. Int J Mol Sci. 2022. PMID: 36555452 Free PMC article. Review.
-
Presence of increased inflammatory infiltrates accompanied by activated dendritic cells in the left atrium in rheumatic heart disease.PLoS One. 2018 Sep 27;13(9):e0203756. doi: 10.1371/journal.pone.0203756. eCollection 2018. PLoS One. 2018. PMID: 30261069 Free PMC article.
References
-
- Cone LA, Woodard DR, Schlievert PM, Tomory GS. Clinical and bacteriology observations of a toxic shock like syndrome due to Streptococcus pyogenes. N Engl J Med. 1987;317:146–9. - PubMed
-
- Ginsburg I. Mechanisms of cell and tissue injury induced by group A streptococci: relation to poststreptococcal sequelae. J Infect Dis. 1972;126:294–301. - PubMed
-
- Kotb M. Role of superantigens in infectious diseases and their sequelae. Curr Opin Infect Dis. 1992;5:364–74.
-
- Drake CG, Kotzin BL. Superantigens: biology, immunology and potential role in disease. J Clin Immunol. 1992;12:149–62. - PubMed
-
- Janeway CJ, Yagi J, Caurad PJ, Katz ME, Jones B, Vroegop S, Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behaviour. Immunol Rev. 1989;107:61–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

