Detection of antibodies directed against the cytoplasmic region of the human acetylcholine receptor in sera from myasthenia gravis patients
- PMID: 10209519
- PMCID: PMC1905210
- DOI: 10.1046/j.1365-2249.1999.00846.x
Detection of antibodies directed against the cytoplasmic region of the human acetylcholine receptor in sera from myasthenia gravis patients
Abstract
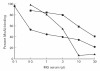
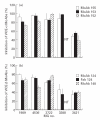
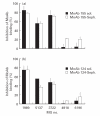
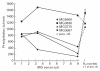
The nicotinic acetylcholine receptor (AChR) is the autoantigen in the human autoimmune disease myasthenia gravis (MG). Anti-AChR antibodies in MG sera bind mainly to conformational epitopes, therefore the determination of their specificities requires the use of native AChR. Antibody competition studies suggest that most MG antibodies are directed against the extracellular part of the molecule, whereas antibodies directed against the cytoplasmic region of the AChR have not been detected. To determine whether even small quantities of such antibodies exist in MG sera, we performed competition experiments based on the inhibition by MG sera of the binding of MoAbs to the human AChR, rather than inhibition by MoAbs of the binding of MG sera performed earlier. When MoAbs directed against cytoplasmic epitopes on the alpha or beta subunits (alpha 373-380 and beta 354-360) were used as test MoAbs, 17% or 9% of MG sera inhibited the binding of the anti-alpha or anti-beta subunit MoAbs, respectively, by > or = 50%. Non-specific inhibition was excluded. These results suggest the presence, in several MG sera, of antibodies directed against cytoplasmic regions of the AChR; yet these antibodies seemed to represent a relatively small proportion of the total anti-AChR antibodies. The corresponding epitopes may be involved in the inducing mechanisms in certain MG cases, and knowledge of the presence of such antibodies may be useful in understanding the autoimmune mechanism involved in MG.
Figures

Similar articles
-
Expression of human-Torpedo hybrid acetylcholine receptor (AChR) for analysing the subunit specificity of antibodies in sera from patients with myasthenia gravis (MG).Clin Exp Immunol. 1997 Sep;109(3):538-46. doi: 10.1046/j.1365-2249.1997.4701367.x. Clin Exp Immunol. 1997. PMID: 9328134 Free PMC article.
-
Fab fragments of monoclonal antibodies protect the human acetylcholine receptor against antigenic modulation caused by myasthenic sera.J Autoimmun. 1989 Dec;2(6):777-89. doi: 10.1016/0896-8411(89)90004-8. J Autoimmun. 1989. PMID: 2619869
-
Future therapeutic strategies in autoimmune myasthenia gravis.Ann N Y Acad Sci. 2003 Sep;998:539-48. doi: 10.1196/annals.1254.071. Ann N Y Acad Sci. 2003. PMID: 14592926
-
On the initial trigger of myasthenia gravis and suppression of the disease by antibodies against the MHC peptide region involved in the presentation of a pathogenic T-cell epitope.Crit Rev Immunol. 2001;21(1-3):1-27. Crit Rev Immunol. 2001. PMID: 11642597 Review.
-
[Antibodies in myasthenia gravis].Rev Neurol (Paris). 2009 Feb;165(2):137-43. doi: 10.1016/j.neurol.2008.11.020. Epub 2009 Jan 21. Rev Neurol (Paris). 2009. PMID: 19162288 Review. French.
Cited by
-
Mechanisms of Autoantibody-Induced Pathology.Front Immunol. 2017 May 31;8:603. doi: 10.3389/fimmu.2017.00603. eCollection 2017. Front Immunol. 2017. PMID: 28620373 Free PMC article. Review.
-
Mapping autoantigen epitopes: molecular insights into autoantibody-associated disorders of the nervous system.J Neuroinflammation. 2016 Aug 30;13(1):219. doi: 10.1186/s12974-016-0678-4. J Neuroinflammation. 2016. PMID: 27577085 Free PMC article. Review.
-
Specific immunotherapy of experimental myasthenia gravis by a novel mechanism.Ann Neurol. 2010 Apr;67(4):441-51. doi: 10.1002/ana.21901. Ann Neurol. 2010. PMID: 20437579 Free PMC article.
References
-
- Drachman DB. Medical progress—myasthenia gravis. N Engl J Med. 1994;330:1797–810. - PubMed
-
- Lindstrom J, Shelton D, Fugii Y. Myasthenia gravis. Adv Immunol. 1988;42:233–84. - PubMed
-
- Galzi JL, Changeux JP. Neurotransmitter-gated ion channels as unconventional allosteric proteins. Curr Opin Struct Biol. 1994;4:554–65.
-
- Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995;373:37–43. - PubMed
-
- Tzartos SJ, Barkas T, Cung MT, et al. Anatomy of the antigenic structure of a large membrane autoantigen, the muscle-type nicotinic acetylcholine receptor. Immunol Rev. 1998;163:89–120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

