Leukemia inhibitory factor augments neurotrophin expression and corticospinal axon growth after adult CNS injury
- PMID: 10212315
- PMCID: PMC6782234
- DOI: 10.1523/JNEUROSCI.19-09-03556.1999
Leukemia inhibitory factor augments neurotrophin expression and corticospinal axon growth after adult CNS injury
Abstract
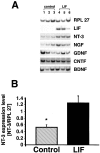
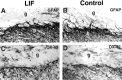
The cytokine leukemia inhibitory factor (LIF) modulates glial and neuronal function in development and after peripheral nerve injury, but little is known regarding its role in the injured adult CNS. To further understand the biological role of LIF and its potential mechanisms of action after CNS injury, effects of cellularly delivered LIF on axonal growth, glial activation, and expression of trophic factors were examined after adult mammalian spinal cord injury. Fibroblasts genetically modified to produce high amounts of LIF were grafted to the injured spinal cords of adult Fischer 344 rats. Two weeks after injury, animals with LIF-secreting cells showed a specific and significant increase in corticospinal axon growth compared with control animals. Furthermore, expression of neurotrophin-3, but not nerve growth factor, brain-derived neurotrophic factor, glia cell line-derived neurotrophic factor, or ciliary neurotrophic factor, was increased at the lesion site in LIF-grafted but not in control subjects. No differences in astroglial and microglial/macrophage activation were observed. Thus, LIF can directly or indirectly modulate molecular and cellular responses of the adult CNS to injury. These findings also demonstrate that neurotrophic molecules can augment expression of other trophic factors in vivo after traumatic injury in the adult CNS.
Figures






References
-
- Aloisi F, Rosa S, Testa U, Bonsi P, Russo G, Peschle C, Levi G. Regulation of leukemia inhibitory factor synthesis in cultured human astrocytes. J Immunol. 1994;152:5022–5031. - PubMed
-
- Apfel SC, Wright DE, Wiideman AM, Dormia C, Snider WD, Kessler JA. Nerve growth factor regulates the expression of brain-derived neurotrophic factor mRNA in the peripheral nervous system. Mol Cell Neurosci. 1996;7:134–142. - PubMed
-
- Banner LR, Moayeri NN, Patterson PH. Leukemia inhibitory factor is expressed in astrocytes following cortical brain injury. Exp Neurol. 1997;147:1–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
